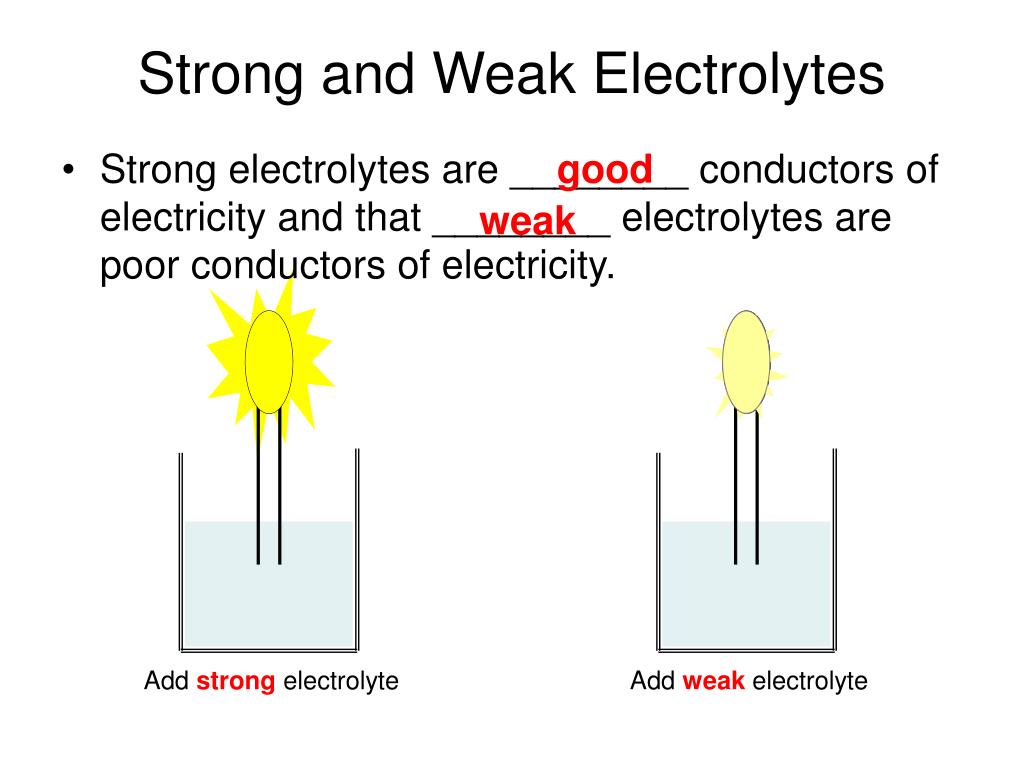
What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity . Strong electrolytes dissociate completely, weak electrolyte dissociate only. When the electrolyte is fused or is dissolved in the solvent, these broken up. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. Electrolytes are substances that form ions in aqueous solutions. The electrolyte can conduct electricity because the molecules are broken up into ions. Define the limiting ionic conductivity, and comment on. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. Explain the distinction between ionic diffusion and ionic migration. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Here’s how to approach this question.
from www.slideserve.com
Explain the distinction between ionic diffusion and ionic migration. Electrolytes are substances that form ions in aqueous solutions. Strong electrolytes dissociate completely, weak electrolyte dissociate only. The electrolyte can conduct electricity because the molecules are broken up into ions. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. Define the limiting ionic conductivity, and comment on. When the electrolyte is fused or is dissolved in the solvent, these broken up. Here’s how to approach this question.
PPT Strong and Weak Electrolytes PowerPoint Presentation, free
What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Here’s how to approach this question. Strong electrolytes dissociate completely, weak electrolyte dissociate only. The electrolyte can conduct electricity because the molecules are broken up into ions. Explain the distinction between ionic diffusion and ionic migration. Electrolytes are substances that form ions in aqueous solutions. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. Here’s how to approach this question. Define the limiting ionic conductivity, and comment on. When the electrolyte is fused or is dissolved in the solvent, these broken up.
From www.aakash.ac.in
Electrolytes and Nonelectrolytes Types, Differences & Examples AESL What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity The electrolyte can conduct electricity because the molecules are broken up into ions. Strong electrolytes dissociate completely, weak electrolyte dissociate only. Electrolytes are substances that form ions in aqueous solutions. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Strong electrolytes dissociate completely, weak electrolyte dissociate only. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. The electrolyte can conduct electricity because the molecules are broken up. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From brainly.in
how does an electrolyte conduct electricity Brainly.in What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. Electrolytes are substances that form ions in aqueous solutions. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Define the limiting ionic conductivity, and comment on. When the electrolyte is fused or is. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Plan for Wed, 1 Oct 08 PowerPoint Presentation ID4479101 What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Electrolytes are substances that form ions in aqueous solutions. Define the limiting ionic conductivity, and comment on. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. When the electrolyte is fused or is dissolved in the solvent, these broken up. Here’s how to approach this question. Strong electrolytes. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Explain the distinction between ionic diffusion and ionic migration. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. Electrolytes are substances that form ions in aqueous solutions. Define the limiting ionic conductivity, and comment on. The electrolyte can conduct electricity because the molecules are broken up into ions.. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.slideshare.net
Introduction to ElectroChemistry What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Define the limiting ionic conductivity, and comment on. Electrolytes are substances that form ions in aqueous solutions. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Explain the distinction between ionic diffusion and ionic migration. The electrolyte can conduct electricity because the molecules are broken up into ions. Here’s. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From study.com
Electrolysis of Aqueous Solutions Lesson What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Explain the distinction between ionic diffusion and ionic migration. Define the limiting ionic conductivity, and comment on. Here’s how to approach this question. Electrolytes are substances that form ions in aqueous solutions. Strong electrolytes dissociate completely, weak electrolyte dissociate only. The electrolyte can conduct electricity because the molecules are broken up into ions. In some cases, we find that solutions. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity When the electrolyte is fused or is dissolved in the solvent, these broken up. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. Strong electrolytes dissociate completely, weak electrolyte dissociate only. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.vrogue.co
Electrolyte Nonelectrolyte Solutions Infographic Diag vrogue.co What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Here’s how to approach this question. Explain the distinction between ionic diffusion and ionic migration. The electrolyte can conduct electricity because the molecules are broken up into ions. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. When the electrolyte is fused or is dissolved in the solvent, these broken up. Electrolytes are. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Electrolytes PowerPoint Presentation, free download ID748165 What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Electrolytes are substances that form ions in aqueous solutions. When the electrolyte is fused or is dissolved in the solvent, these broken up. Here’s how to approach this question. Define the limiting ionic conductivity, and comment on. The electrolyte can conduct electricity because the molecules are broken up into ions. Explain the distinction between ionic diffusion and ionic migration. In. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.vrogue.co
Electrolyte Nonelectrolyte Solutions Infographic Diag vrogue.co What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Strong electrolytes dissociate completely, weak electrolyte dissociate only. When the electrolyte is fused or is dissolved in the solvent, these broken up. Explain the distinction between ionic diffusion and ionic migration. Electrolytes are substances that form ions in aqueous solutions. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to.. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.chegg.com
Solved uestion 8 of 20> What is the best explanation for why What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. Define the limiting ionic conductivity, and comment on. Here’s how to approach this question. Explain the distinction between ionic diffusion and ionic migration.. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity The electrolyte can conduct electricity because the molecules are broken up into ions. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. Strong electrolytes dissociate completely, weak electrolyte dissociate only. Electrolytes are substances that form ions in aqueous solutions. Define the limiting ionic conductivity, and comment on. In. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.chegg.com
Solved Which of the following best explains why electrolyte What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Strong electrolytes dissociate completely, weak electrolyte dissociate only. When the electrolyte is fused or is dissolved in the solvent, these broken up. Here’s how to approach this question. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. In some cases, we find that solutions prepared from covalent compounds conduct. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.youtube.com
Which of the following best explains why electrolytes solutions conduct What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity When the electrolyte is fused or is dissolved in the solvent, these broken up. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. To address why electrolyte solutions conduct electricity, consider the role. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Strong and Weak Electrolytes PowerPoint Presentation, free What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Define the limiting ionic conductivity, and comment on. Here’s how to approach this question. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. Electrolytes are substances that form ions in aqueous solutions. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Strong electrolytes dissociate completely, weak electrolyte dissociate only. Explain the distinction between ionic diffusion and ionic migration. When the electrolyte is fused or is dissolved in the solvent, these broken up. Electrolytes are substances that form ions in aqueous solutions.. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.electricity-magnetism.org
How do electrolytes conduct electricity? What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. Explain the distinction between ionic diffusion and ionic migration. Here’s how to approach this question. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. In some cases, solutions prepared from covalent compounds. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From energyeducation.ca
Electrolyte Energy Education What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Electrolytes are substances that form ions in aqueous solutions. Here’s how to approach this question. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. Explain the distinction between. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.pinterest.com
Electrolyte Easy Science Electrolytes, Easy science, Flashcards What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity The electrolyte can conduct electricity because the molecules are broken up into ions. Explain the distinction between ionic diffusion and ionic migration. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. In. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.yourdictionary.com
Examples of Electrolytes Basic Explanation and Purpose YourDictionary What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Explain the distinction between ionic diffusion and ionic migration. Here’s how to approach this question. Define the limiting ionic. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Chemistry 101 Chap. 4 PowerPoint Presentation, free download What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Define the limiting ionic conductivity, and comment on. Here’s how to approach this question. Electrolytes are substances that form ions in aqueous solutions. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From quizdbbarnstorms.z21.web.core.windows.net
What Is Electrolyte And Nonelectrolyte What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Here’s how to approach this question. Define the limiting ionic conductivity, and comment on. When the electrolyte is fused or is dissolved in the solvent, these broken up. Explain the distinction between ionic diffusion and ionic migration. Strong electrolytes dissociate completely, weak electrolyte dissociate only. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Explain the distinction between ionic diffusion and ionic migration. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. Electrolytes are substances that form ions in aqueous solutions. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. In. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.youtube.com
Electrolytes Definition, Examples, & Practice YouTube What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Explain the distinction between ionic diffusion and ionic migration. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. Electrolytes are substances that form ions in aqueous solutions. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Here’s how to approach this question.. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity When the electrolyte is fused or is dissolved in the solvent, these broken up. Strong electrolytes dissociate completely, weak electrolyte dissociate only. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. The electrolyte can conduct electricity because the molecules are broken up into ions. In some cases, we find. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From in.pinterest.com
What is Electrolysis and Electrolyte with examples. Science fair What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Define the limiting ionic conductivity, and comment on. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Electrolytes are substances that form ions in aqueous solutions. To address why electrolyte solutions conduct electricity,. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Explain the distinction between ionic diffusion and ionic migration. Strong electrolytes dissociate completely, weak electrolyte dissociate only. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. The electrolyte can conduct electricity because the molecules are broken up into ions. Here’s how to approach this question. When the electrolyte is fused or is dissolved. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID5813936 What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. To address why electrolyte solutions conduct electricity, consider the role of ions dissociated in the solution due to the potential difference. Explain the distinction between ionic diffusion and ionic migration. Define the limiting ionic conductivity, and comment on. Electrolytes are. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.youtube.com
Electrolysis and Electrical Conductance Conductance of Electrolytes What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Explain the distinction between ionic diffusion and ionic migration. Strong electrolytes dissociate completely, weak electrolyte dissociate only. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. The electrolyte can conduct electricity because the molecules are broken up into ions. When the electrolyte is fused or is dissolved in the solvent, these broken up.. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Electrolytes PowerPoint Presentation, free download ID181760 What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity When the electrolyte is fused or is dissolved in the solvent, these broken up. The electrolyte can conduct electricity because the molecules are broken up into ions. Electrolytes are substances that form ions in aqueous solutions. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Strong electrolytes dissociate completely,. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.teachoo.com
Do liquids conduct electricity? Class 8 Science Notes Teachoo What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Explain the distinction between ionic diffusion and ionic migration. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Define the limiting ionic conductivity, and comment on. Strong electrolytes dissociate completely, weak electrolyte dissociate only. Electrolytes are substances that form ions in aqueous solutions. Here’s how to approach this question.. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity When the electrolyte is fused or is dissolved in the solvent, these broken up. Explain the distinction between ionic diffusion and ionic migration. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Strong electrolytes dissociate completely, weak electrolyte dissociate only. In some cases, we find that solutions prepared from. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. Explain the distinction between ionic diffusion and ionic migration. The electrolyte can conduct electricity because the molecules are broken up into ions. Here’s how to approach this question. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.
From www.numerade.com
SOLVED What is the best explanation for why electrolyte solutions What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity Define the limiting ionic conductivity, and comment on. Here’s how to approach this question. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute. To address why electrolyte solutions conduct electricity, consider the role. What Is The Best Explanation For Why Electrolyte Solutions Conduct Electricity.