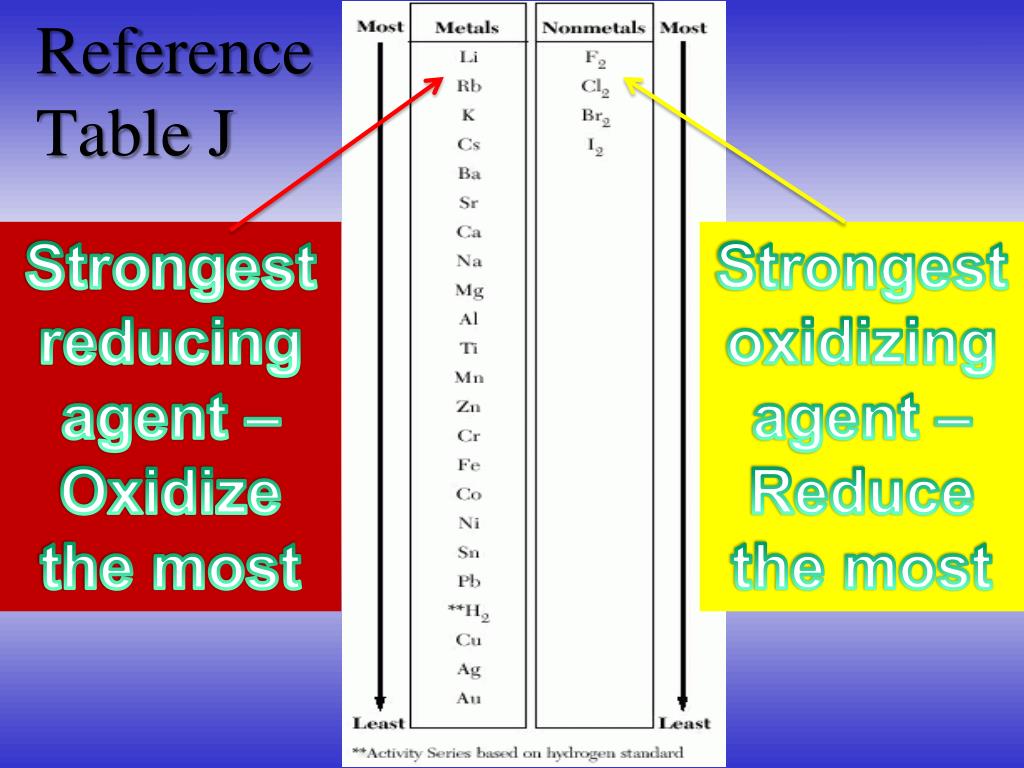
Which Metal Is The Best Oxidizing Agent . F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. You rank oxidizing agents according to their standard. The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. Which species—sn 4+ (aq), cl − (aq), ag + (aq), cr 3+ (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution.
from www.slideserve.com
F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. Which species—sn 4+ (aq), cl − (aq), ag + (aq), cr 3+ (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. You rank oxidizing agents according to their standard. The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+.
PPT Oxidizing & Reducing Agents PowerPoint Presentation, free
Which Metal Is The Best Oxidizing Agent F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. You rank oxidizing agents according to their standard. The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. Which species—sn 4+ (aq), cl − (aq), ag + (aq), cr 3+ (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution.
From www.slideserve.com
PPT Chapter 6 OxidationReduction Reactions PowerPoint Presentation Which Metal Is The Best Oxidizing Agent Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. You rank oxidizing agents according to their standard. Which species—sn 4+ (aq), cl − (aq), ag + (aq), cr 3+ (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in. Which Metal Is The Best Oxidizing Agent.
From www.slideserve.com
PPT Chapter 18 Electrochemistry PowerPoint Presentation, free Which Metal Is The Best Oxidizing Agent The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h. Which Metal Is The Best Oxidizing Agent.
From www.pw.live
List of Strongest Oxidizing agent Physics Wallah Which Metal Is The Best Oxidizing Agent Which species—sn 4+ (aq), cl − (aq), ag + (aq), cr 3+ (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). You rank oxidizing agents according to their standard. The strongest oxidizing agent. Which Metal Is The Best Oxidizing Agent.
From www.chegg.com
Solved Which metal in this activity series is the strongest Which Metal Is The Best Oxidizing Agent F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). You rank oxidizing agents according to their standard. Which species—sn 4+ (aq), cl − (aq), ag +. Which Metal Is The Best Oxidizing Agent.
From www.pw.live
List of Strongest Oxidizing agent Physics Wallah Which Metal Is The Best Oxidizing Agent A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. The strongest oxidizing agent in the list is f2, followed by h2o2,. Which Metal Is The Best Oxidizing Agent.
From chemistrytalk.org
Common Oxidizing Agents & Reducing Agents ChemTalk Which Metal Is The Best Oxidizing Agent F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. You rank oxidizing agents according to their standard. The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. Which species—sn 4 + (aq), cl − (aq), ag. Which Metal Is The Best Oxidizing Agent.
From www.expii.com
Oxidizing and Reducing Agents — Definition & Examples Expii Which Metal Is The Best Oxidizing Agent The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. The strongest oxidizing agent in the list is f2, followed. Which Metal Is The Best Oxidizing Agent.
From www.numerade.com
SOLVEDWhich metal cation is the best oxidizing agent? a. Pb2+ b. Cr3 Which Metal Is The Best Oxidizing Agent Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. Which species—sn 4+ (aq), cl − (aq), ag + (aq), cr 3+ (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. You rank oxidizing agents according to. Which Metal Is The Best Oxidizing Agent.
From www.slideserve.com
PPT REDOX REACTIONS PowerPoint Presentation, free download ID5527873 Which Metal Is The Best Oxidizing Agent The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. Which species—sn 4+ (aq), cl − (aq), ag + (aq), cr 3+ (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. A reducing agent is typically in one of its lower possible oxidation. Which Metal Is The Best Oxidizing Agent.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID6961913 Which Metal Is The Best Oxidizing Agent F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. You rank oxidizing agents according to their standard. The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. A reducing agent is typically in one of its. Which Metal Is The Best Oxidizing Agent.
From www.slideserve.com
PPT REDOX REACTIONS PowerPoint Presentation, free download ID5527873 Which Metal Is The Best Oxidizing Agent You rank oxidizing agents according to their standard. F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. A reducing agent. Which Metal Is The Best Oxidizing Agent.
From slidetodoc.com
OXIDIZING AND REDUCING AGENTS OXIDIZING AND REDUCING AGENTS Which Metal Is The Best Oxidizing Agent The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). You rank oxidizing agents according to their standard. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Which species—sn 4+ (aq), cl − (aq), ag + (aq),. Which Metal Is The Best Oxidizing Agent.
From www.numerade.com
SOLVED Which species is the best oxidizing agent? Potassium metal Which Metal Is The Best Oxidizing Agent Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). A reducing agent is typically in one of its lower. Which Metal Is The Best Oxidizing Agent.
From www.chegg.com
Solved 4. Which metal cation is the best oxidizing agent? Which Metal Is The Best Oxidizing Agent Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. A reducing agent is typically in one of its lower. Which Metal Is The Best Oxidizing Agent.
From www.pw.live
List of Strongest Oxidizing agent Physics Wallah Which Metal Is The Best Oxidizing Agent You rank oxidizing agents according to their standard. The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. F 2 is such a good oxidizing agent that metals,. Which Metal Is The Best Oxidizing Agent.
From www.numerade.com
SOLVED Vanadium is a transition metal that can be found in the four Which Metal Is The Best Oxidizing Agent The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst. Which Metal Is The Best Oxidizing Agent.
From trace-well-warren.blogspot.com
Which One of the Following Is the Best Oxidizing Agent Which Metal Is The Best Oxidizing Agent Which species—sn 4+ (aq), cl − (aq), ag + (aq), cr 3+ (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. F 2 is such a good oxidizing agent that metals, quartz, asbestos,. Which Metal Is The Best Oxidizing Agent.
From www.slideserve.com
PPT Oxidizing & Reducing Agents PowerPoint Presentation, free Which Metal Is The Best Oxidizing Agent Which species—sn 4+ (aq), cl − (aq), ag + (aq), cr 3+ (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. F 2 is such a good. Which Metal Is The Best Oxidizing Agent.
From www.chem.fsu.edu
Lab Purpose Which Metal Is The Best Oxidizing Agent Which species—sn 4+ (aq), cl − (aq), ag + (aq), cr 3+ (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. You rank oxidizing agents according to their standard. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Which species—sn 4 + (aq), cl. Which Metal Is The Best Oxidizing Agent.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID3527121 Which Metal Is The Best Oxidizing Agent F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. You rank oxidizing agents according to their standard. The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). Which species—sn 4 + (aq), cl − (aq), ag. Which Metal Is The Best Oxidizing Agent.
From www.slideserve.com
PPT Relative Strengths of Oxidizing and Reducing Agents PowerPoint Which Metal Is The Best Oxidizing Agent The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. A reducing agent is typically in one of its lower possible oxidation states, and is known as. Which Metal Is The Best Oxidizing Agent.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281515 Which Metal Is The Best Oxidizing Agent A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. You rank oxidizing agents according to their standard. The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). F 2 is such a good oxidizing agent that metals,. Which Metal Is The Best Oxidizing Agent.
From chem.libretexts.org
10.6.2. Strong Oxidizing Agents Chemistry LibreTexts Which Metal Is The Best Oxidizing Agent A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2. Which Metal Is The Best Oxidizing Agent.
From www.chegg.com
Solved Which of the following is the best oxidizing agent Which Metal Is The Best Oxidizing Agent The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). You rank oxidizing agents according to their standard. Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. Which species—sn. Which Metal Is The Best Oxidizing Agent.
From www.slideserve.com
PPT Redox Oxidation and Reduction PowerPoint Presentation, free Which Metal Is The Best Oxidizing Agent A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2. Which Metal Is The Best Oxidizing Agent.
From www.chegg.com
Solved Best oxidizing agent Best reducing agent Which Metal Is The Best Oxidizing Agent A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2. Which Metal Is The Best Oxidizing Agent.
From www.vedantu.com
The element that is the best oxidizing agent isA) CaB) AuC) HD) Fe Which Metal Is The Best Oxidizing Agent The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to. Which Metal Is The Best Oxidizing Agent.
From www.researchgate.net
Distribution of the oxidizing agent concentration in a solution flowing Which Metal Is The Best Oxidizing Agent The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. Which species—sn 4+ (aq), cl − (aq), ag + (aq), cr 3+ (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. A reducing agent is typically in one of its lower possible oxidation. Which Metal Is The Best Oxidizing Agent.
From www.numerade.com
SOLVEDWhich metal cation is the best oxidizing agent? a. Pb^2+ b. C r Which Metal Is The Best Oxidizing Agent You rank oxidizing agents according to their standard. The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. A reducing agent is typically in one of its. Which Metal Is The Best Oxidizing Agent.
From slidetodoc.com
Oxidizing Reducing Agents Oxidizing Agents An oxidizing agent Which Metal Is The Best Oxidizing Agent Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. The strongest oxidizing agent in the list is f2, followed by. Which Metal Is The Best Oxidizing Agent.
From chemistry.stackexchange.com
chemistry Best oxidizing and reducing agents Na, Zn^2+, Ba Which Metal Is The Best Oxidizing Agent A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). Which species—sn 4+ (aq), cl − (aq), ag + (aq), cr 3+ (aq), and/or h 2 o 2. Which Metal Is The Best Oxidizing Agent.
From www.slideserve.com
PPT Relative Strengths of Oxidizing and Reducing Agents PowerPoint Which Metal Is The Best Oxidizing Agent The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. F 2 is such a good oxidizing agent that metals, quartz, asbestos, and even water burst into flame in its presence. Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h. Which Metal Is The Best Oxidizing Agent.
From www.chegg.com
Solved explain which metal is the easiest to oxidize? which Which Metal Is The Best Oxidizing Agent The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). The strongest oxidizing agent in the list is f2, followed by h2o2, and so on down to the weakest oxidizing agent, li+. Which species—sn 4+ (aq), cl − (aq), ag + (aq), cr 3+ (aq), and/or h 2. Which Metal Is The Best Oxidizing Agent.
From slidetodoc.com
OXIDIZING AND REDUCING AGENTS OXIDIZING AND REDUCING AGENTS Which Metal Is The Best Oxidizing Agent Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. The best oxidising agent without doubt is zinc(ii) as it is much more noble than barium (although still not a noble metal). Which species—sn 4+ (aq), cl − (aq), ag + (aq),. Which Metal Is The Best Oxidizing Agent.
From protonstalk.com
Electrochemical Series Features, Applications, Examples ProtonsTalk Which Metal Is The Best Oxidizing Agent Which species—sn 4+ (aq), cl − (aq), ag + (aq), cr 3+ (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. Which species—sn 4 + (aq), cl − (aq), ag + (aq), cr 3 + (aq), and/or h 2 o 2 (aq)—is the strongest oxidizing agent in aqueous solution. The strongest oxidizing agent in the. Which Metal Is The Best Oxidizing Agent.