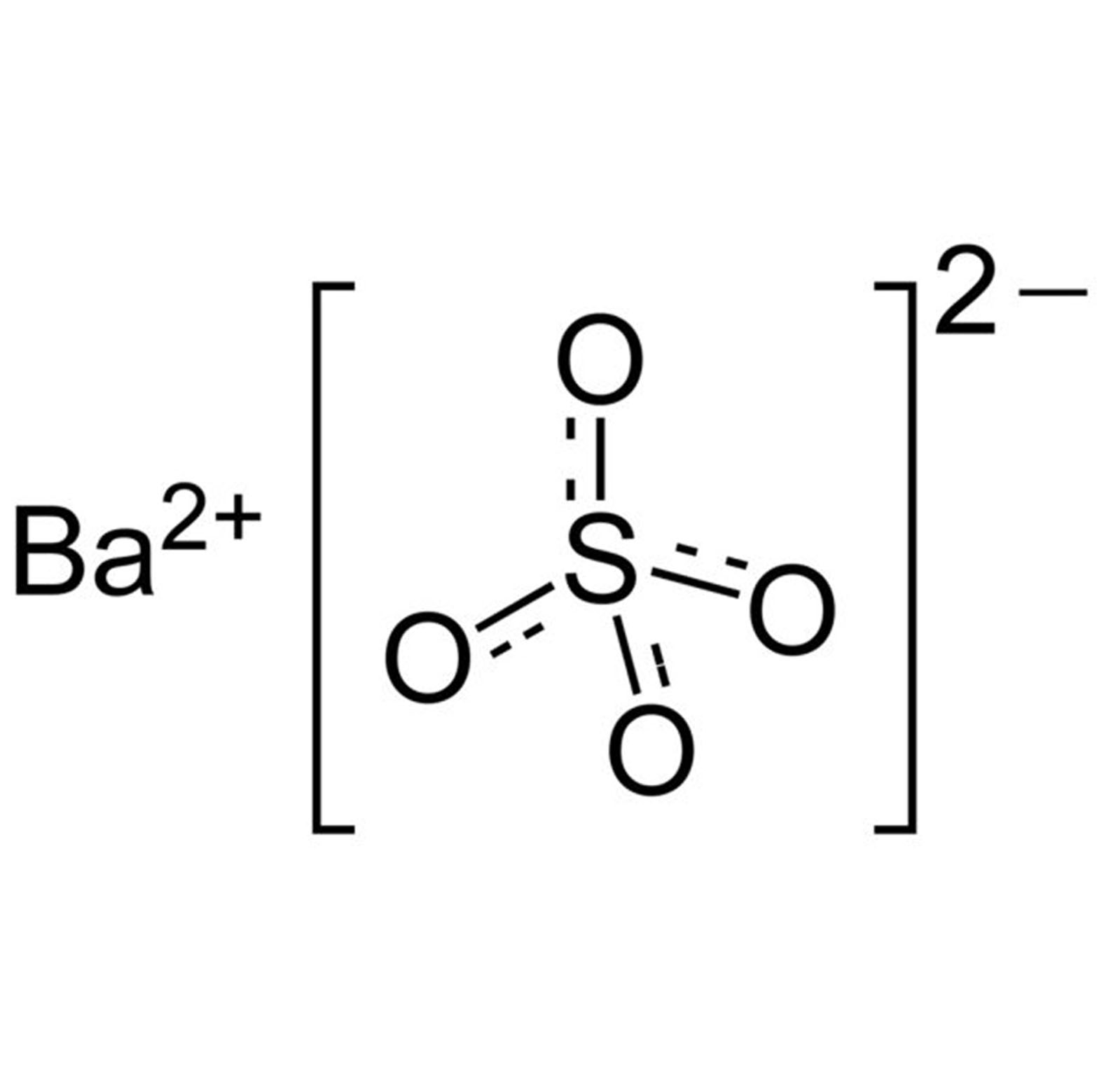
Magnesium Sulfate Equation . Magnesium + copper (ii) sulfate → magnesium sulfate + copper. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. This is the word equation: Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to produce a solution of magnesium sulfate, plus liquid water and some carbon dioxide. Magnesium sulfate is a white crystalline substance which can exist in several hydrated forms. The most common form of this.
from www.animalia-life.club
Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. Magnesium + copper (ii) sulfate → magnesium sulfate + copper. The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. The most common form of this. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to produce a solution of magnesium sulfate, plus liquid water and some carbon dioxide. Magnesium sulfate is a white crystalline substance which can exist in several hydrated forms. This is the word equation:
Magnesium Sulfate Lewis Structure
Magnesium Sulfate Equation A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. Magnesium + copper (ii) sulfate → magnesium sulfate + copper. A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. This is the word equation: Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to produce a solution of magnesium sulfate, plus liquid water and some carbon dioxide. Magnesium sulfate is a white crystalline substance which can exist in several hydrated forms. The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. The most common form of this. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air.
From brainly.in
formula of magnesium sulphate by criss cross method Brainly.in Magnesium Sulfate Equation Magnesium sulfate is a white crystalline substance which can exist in several hydrated forms. Magnesium + copper (ii) sulfate → magnesium sulfate + copper. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. This is the word equation: Mg (s) + cuso 4 (aq) → mgso 4 (aq). Magnesium Sulfate Equation.
From www.fishersci.ca
Magnesium Sulfate Anhydrous (Powder/Certified), Fisher Chemical Magnesium Sulfate Equation Magnesium + copper (ii) sulfate → magnesium sulfate + copper. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. The most common form of this. A hydrate form of magnesium sulfate called kieserite,. Magnesium Sulfate Equation.
From dayanara-blogmosley.blogspot.com
Magnesium Sulfate and Sodium Carbonate Balanced Equation Magnesium Sulfate Equation Magnesium sulfate is a white crystalline substance which can exist in several hydrated forms. Magnesium + copper (ii) sulfate → magnesium sulfate + copper. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. This is the word equation: The most common form of this. The symbol equation shows. Magnesium Sulfate Equation.
From www.numerade.com
SOLVED Consider the double replacement reactions. The reaction of Magnesium Sulfate Equation The most common form of this. Magnesium + copper (ii) sulfate → magnesium sulfate + copper. The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to produce a solution of magnesium sulfate, plus liquid water and some carbon. Magnesium Sulfate Equation.
From testbook.com
Magnesium Sulphate Formula Know Structure, Properties and Uses Magnesium Sulfate Equation The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. The most common form of this. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. Magnesium + copper (ii) sulfate → magnesium sulfate + copper. Magnesium sulfate is a white crystalline substance which can exist in several hydrated. Magnesium Sulfate Equation.
From www.chemistryworld.com
Magnesium sulfate Podcast Chemistry World Magnesium Sulfate Equation The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. Magnesium sulfate is a white crystalline substance which can exist in several hydrated forms. The most common form of this. A hydrate form. Magnesium Sulfate Equation.
From www.vrogue.co
Magnesium Sulfate Formula Chemical Formula Of Magnesi vrogue.co Magnesium Sulfate Equation Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to produce a solution of magnesium sulfate, plus liquid water and some carbon dioxide. A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a. Magnesium Sulfate Equation.
From www.youtube.com
How to Write the Formula for Magnesium sulfate heptahydrate YouTube Magnesium Sulfate Equation The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. The most common form of this. This is the word equation: Magnesium sulfate is a white crystalline substance which can exist in several hydrated forms. Magnesium + copper (ii) sulfate → magnesium sulfate + copper. The symbol equation shows that solid magnesium carbonate reacts. Magnesium Sulfate Equation.
From www.animalia-life.club
Magnesium Sulfate Lewis Structure Magnesium Sulfate Equation Magnesium + copper (ii) sulfate → magnesium sulfate + copper. This is the word equation: The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. Magnesium sulfate is a white crystalline substance which. Magnesium Sulfate Equation.
From www.youtube.com
How to Write the Formula for Magnesium sulfite YouTube Magnesium Sulfate Equation A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. Magnesium sulfate is a white crystalline substance which can exist in several hydrated forms. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. The magnesium displaces the copper, and the. Magnesium Sulfate Equation.
From www.coursehero.com
[Solved] Prescribed is Magnesium Sulfate 6 grams, to be infused in 30 Magnesium Sulfate Equation A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. This is the word equation: The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to produce a solution of magnesium sulfate, plus liquid water and some carbon dioxide. The most common form of this. Magnesium sulfate. Magnesium Sulfate Equation.
From www.toppr.com
The equation shows the reaction between magnesium and sulphuric acid Magnesium Sulfate Equation Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to produce a solution of magnesium sulfate, plus liquid water and some carbon dioxide. The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate.. Magnesium Sulfate Equation.
From www.youtube.com
How to Write the Formula for Magnesium sulfate YouTube Magnesium Sulfate Equation This is the word equation: Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. Magnesium sulfate, mgso 4, is a. Magnesium Sulfate Equation.
From www.youtube.com
Equation for MgSO4 + H2O (Magnesium sulfate + Water) YouTube Magnesium Sulfate Equation This is the word equation: The most common form of this. A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide. Magnesium Sulfate Equation.
From www.shutterstock.com
Magnesium Sulfate Formula Mgso4 Mgo4s Often ilustrações stock Magnesium Sulfate Equation A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. This is the word equation: Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. The symbol equation shows that solid. Magnesium Sulfate Equation.
From www.dreamstime.com
Magnesium Sulfate, Chemical Structure. Many Uses Include As Drug To Magnesium Sulfate Equation The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. Magnesium + copper (ii) sulfate → magnesium sulfate + copper. The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to. Magnesium Sulfate Equation.
From animalia-life.club
Magnesium Sulfate Lewis Structure Magnesium Sulfate Equation Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. Magnesium + copper (ii) sulfate →. Magnesium Sulfate Equation.
From www.sciencephoto.com
Magnesium sulfate, chemical structure, illustration Stock Image Magnesium Sulfate Equation Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. Magnesium + copper (ii) sulfate → magnesium sulfate + copper. The most common form of this. A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. The magnesium displaces the copper,. Magnesium Sulfate Equation.
From www.animalia-life.club
Magnesium Sulfate Lewis Structure Magnesium Sulfate Equation Magnesium sulfate is a white crystalline substance which can exist in several hydrated forms. Magnesium + copper (ii) sulfate → magnesium sulfate + copper. This is the word equation: Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to. Magnesium Sulfate Equation.
From studylib.net
Determination of the formula of hydrated magnesium sulfate Activity Magnesium Sulfate Equation This is the word equation: The most common form of this. The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to produce a solution of magnesium sulfate, plus liquid water and some carbon dioxide. Mg (s) + cuso. Magnesium Sulfate Equation.
From www.sciencephoto.com
Magnesium sulfate, chemical structure, illustration Stock Image Magnesium Sulfate Equation Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. Magnesium + copper (ii) sulfate →. Magnesium Sulfate Equation.
From stock.adobe.com
Molecular formula and chemical structure of magnesium sulfate vector de Magnesium Sulfate Equation The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. Mg (s) + cuso 4. Magnesium Sulfate Equation.
From www.istockphoto.com
Magnesium Sulfate Formula Mgso4 Or Mgo4s It Is Often Encountered As The Magnesium Sulfate Equation Magnesium + copper (ii) sulfate → magnesium sulfate + copper. This is the word equation: Magnesium sulfate is a white crystalline substance which can exist in several hydrated forms. The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this.. Magnesium Sulfate Equation.
From www.alamy.com
3D image of Magnesium sulfate skeletal formula molecular chemical Magnesium Sulfate Equation Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to produce a solution of magnesium sulfate, plus liquid water and some carbon dioxide. The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate.. Magnesium Sulfate Equation.
From www.dreamstime.com
Magnesium Sulfate, Salt, Molecular Structures, 3d Model, Structural Magnesium Sulfate Equation The most common form of this. The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to produce a solution of magnesium sulfate, plus liquid water and some carbon dioxide. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. The magnesium displaces the copper, and the products are copper. Magnesium Sulfate Equation.
From biobasic-asia.com
Magnesium Sulfate, anhydrous Bio Basic Asia Pacific Pte Ltd Magnesium Sulfate Equation Magnesium + copper (ii) sulfate → magnesium sulfate + copper. This is the word equation: The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of. Magnesium Sulfate Equation.
From www.dreamstime.com
3D Image of Magnesium Sulfate Skeletal Formula Stock Illustration Magnesium Sulfate Equation Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. Magnesium sulfate is a white crystalline substance which. Magnesium Sulfate Equation.
From www.alamy.com
Magnesium sulfate molecule. It is is an salt and Magnesium Sulfate Equation The most common form of this. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. A hydrate. Magnesium Sulfate Equation.
From www.alamy.com
Magnesium sulfate, chemical structure. Many uses include as drug to Magnesium Sulfate Equation Magnesium + copper (ii) sulfate → magnesium sulfate + copper. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. The magnesium displaces the copper, and the products are copper and a solution of. Magnesium Sulfate Equation.
From www.smartscience.co.th
Magnesium sulfate, anhydrous smartscience Magnesium Sulfate Equation A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. Magnesium sulfate is a white crystalline substance which can exist in several hydrated forms. This is the word equation: The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. Magnesium + copper (ii) sulfate → magnesium. Magnesium Sulfate Equation.
From www.youtube.com
Net Ionic Equation for MgSO4 + BaCl2 (Magnesium sulfate and Barium Magnesium Sulfate Equation The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. A hydrate form of magnesium sulfate called kieserite, mgso 4 ∙h 2 o, occurs as a mineral deposit. Magnesium sulfate is a white crystalline substance which can exist in several hydrated forms. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu. Magnesium Sulfate Equation.
From www.toppr.com
The equation shows the reaction between magnesium and sulphuric acidMg Magnesium Sulfate Equation This is the word equation: The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to produce a solution of magnesium sulfate, plus liquid water and some carbon dioxide. The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. A hydrate form of magnesium sulfate called kieserite, mgso 4. Magnesium Sulfate Equation.
From www.numerade.com
SOLVED Magnesium reacts with sulfuric acid according to the following Magnesium Sulfate Equation The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to produce a solution of magnesium sulfate, plus liquid water and some carbon dioxide. The most common form of this. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. Magnesium sulfate is a white crystalline substance which can exist. Magnesium Sulfate Equation.
From brainly.in
Molecular formula of magnesium sulphate by crisscross method Brainly.in Magnesium Sulfate Equation Magnesium sulfate, mgso 4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. Magnesium sulfate is a white crystalline substance which can exist in several hydrated forms. This is the word equation: The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate. Magnesium + copper (ii). Magnesium Sulfate Equation.
From stock.adobe.com
Magnesium Sulfate MgSO4 molecule. Simple molecular formula consisting Magnesium Sulfate Equation Magnesium sulfate is a white crystalline substance which can exist in several hydrated forms. Mg (s) + cuso 4 (aq) → mgso 4 (aq) + cu (s) in this. The symbol equation shows that solid magnesium carbonate reacts with a solution of sulfuric acid to produce a solution of magnesium sulfate, plus liquid water and some carbon dioxide. A hydrate. Magnesium Sulfate Equation.