Potassium Sulfide And Zinc Bromide Precipitate . Place about 5cm 3 of the solution into a test tube. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Add a few drops of sodium hydroxide solution. Enter an equation of an ionic chemical equation and press the balance button. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. If a reaction occurs and a. Dissolve a small quantity of the substance in water. How many grams of zinc(ii) nitrate and sodium sulfide. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate.
from us.metoree.com
Place about 5cm 3 of the solution into a test tube. How many grams of zinc(ii) nitrate and sodium sulfide. Add a few drops of sodium hydroxide solution. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Dissolve a small quantity of the substance in water. Enter an equation of an ionic chemical equation and press the balance button. If a reaction occurs and a. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate.
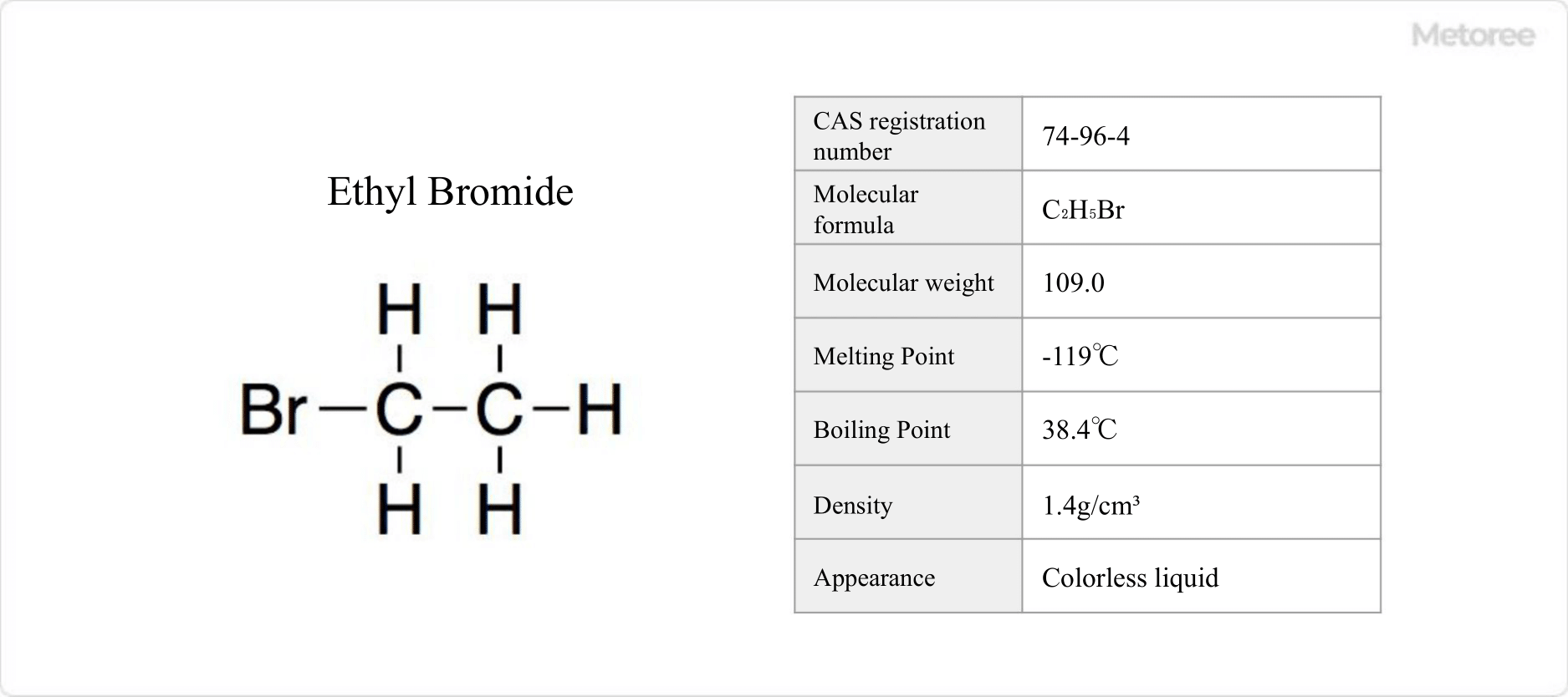
41 Ethyl Bromide Manufacturers in 2024 Metoree
Potassium Sulfide And Zinc Bromide Precipitate Enter an equation of an ionic chemical equation and press the balance button. Enter an equation of an ionic chemical equation and press the balance button. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Place about 5cm 3 of the solution into a test tube. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Add a few drops of sodium hydroxide solution. How many grams of zinc(ii) nitrate and sodium sulfide. Dissolve a small quantity of the substance in water. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. If a reaction occurs and a. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate.
From www.chemkits.eu
Potassium bromide, 98.5, 7758023 Potassium Sulfide And Zinc Bromide Precipitate Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. If a reaction occurs and a. How many grams of zinc(ii) nitrate and sodium sulfide. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Dissolve a. Potassium Sulfide And Zinc Bromide Precipitate.
From testbook.com
Sodium Bromide Learn Definition, Properties, Structure & Uses Potassium Sulfide And Zinc Bromide Precipitate How many grams of zinc(ii) nitrate and sodium sulfide. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Add a few drops of sodium hydroxide solution. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. If a reaction occurs and a.. Potassium Sulfide And Zinc Bromide Precipitate.
From www.numerade.com
SOLVED Write the net ionic equation for the precipitation reaction Potassium Sulfide And Zinc Bromide Precipitate Place about 5cm 3 of the solution into a test tube. Dissolve a small quantity of the substance in water. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Add a few drops of sodium hydroxide solution. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced. Potassium Sulfide And Zinc Bromide Precipitate.
From www.numerade.com
SOLVED Does a empirical formula of precipitate solution A solution B Potassium Sulfide And Zinc Bromide Precipitate A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Dissolve a small quantity of the substance in water. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Enter an equation of an ionic chemical equation. Potassium Sulfide And Zinc Bromide Precipitate.
From www.numerade.com
SOLVED Complete the table below bv deciding whether precipitate forms Potassium Sulfide And Zinc Bromide Precipitate Dissolve a small quantity of the substance in water. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Two important uses of precipitation reactions are to. Potassium Sulfide And Zinc Bromide Precipitate.
From www.numerade.com
SOLVED Complete the table below by deciding whether a precipitate Potassium Sulfide And Zinc Bromide Precipitate Enter an equation of an ionic chemical equation and press the balance button. Place about 5cm 3 of the solution into a test tube. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Dissolve a small quantity of the substance in water. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may. Potassium Sulfide And Zinc Bromide Precipitate.
From www.numerade.com
SOLVED Texts Complete the table below by deciding whether a Potassium Sulfide And Zinc Bromide Precipitate A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. If a reaction occurs and a. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores. Potassium Sulfide And Zinc Bromide Precipitate.
From www.numerade.com
SOLVED When 12.0 mL of a 6.54x10=4 M zinc bromide solution is combined Potassium Sulfide And Zinc Bromide Precipitate Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Dissolve a small quantity of the substance in water. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Predict the result of mixing reasonably concentrated solutions of. Potassium Sulfide And Zinc Bromide Precipitate.
From www.universalmedicalinc.com
IBI IB40075 Ethidium Bromide Solution 10mL Potassium Sulfide And Zinc Bromide Precipitate The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Dissolve a small quantity of the substance in water. Place about 5cm 3 of the solution into a test tube. If a reaction occurs and a. Predict the result of mixing reasonably concentrated. Potassium Sulfide And Zinc Bromide Precipitate.
From www.sigmaaldrich.co.th
THIAZOLYL BLUE TETRAZOLIU M BROMIDE, 98 Merck Life Sciences Thailand Potassium Sulfide And Zinc Bromide Precipitate Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Enter an equation of an ionic chemical equation and press the balance button. Add a few drops of sodium hydroxide solution. Dissolve a small quantity of the substance in water. Place about 5cm 3 of the solution into a test tube. The precipitate is then removed by. Potassium Sulfide And Zinc Bromide Precipitate.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Potassium Sulfide And Zinc Bromide Precipitate The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Predict the result of mixing reasonably concentrated solutions of. Potassium Sulfide And Zinc Bromide Precipitate.
From www.chegg.com
Solved Predicting precipitation Complete the table below by Potassium Sulfide And Zinc Bromide Precipitate Add a few drops of sodium hydroxide solution. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Enter an equation of an ionic chemical equation and press the balance. Potassium Sulfide And Zinc Bromide Precipitate.
From www.sciencecompany.com
Reagent grade Potassium Bromide, 500g for sale. Buy from The Science Potassium Sulfide And Zinc Bromide Precipitate The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. A typical. Potassium Sulfide And Zinc Bromide Precipitate.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Potassium Sulfide And Zinc Bromide Precipitate Enter an equation of an ionic chemical equation and press the balance button. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00. Potassium Sulfide And Zinc Bromide Precipitate.
From www.sciencephoto.com
Potassium Bromide (KBr) Stock Image C030/7401 Science Photo Library Potassium Sulfide And Zinc Bromide Precipitate The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover. Potassium Sulfide And Zinc Bromide Precipitate.
From www.chegg.com
Solved solution A solution B Does a precipitate form when A Potassium Sulfide And Zinc Bromide Precipitate Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. How many grams of zinc(ii) nitrate and sodium sulfide. If a reaction occurs and a. Place about 5cm 3 of the solution into a test tube. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Dissolve a small. Potassium Sulfide And Zinc Bromide Precipitate.
From www.grainger.com
7758023, 119, Potassium Bromide, Crystal, Reagent, ACS 6MMU1P1220 Potassium Sulfide And Zinc Bromide Precipitate Dissolve a small quantity of the substance in water. If a reaction occurs and a. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Place about 5cm 3 of the solution into a test tube. A typical precipitation. Potassium Sulfide And Zinc Bromide Precipitate.
From www.numerade.com
SOLVED Complete the table below by deciding whether precipitate forms Potassium Sulfide And Zinc Bromide Precipitate Enter an equation of an ionic chemical equation and press the balance button. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Dissolve a small quantity. Potassium Sulfide And Zinc Bromide Precipitate.
From www.youtube.com
Does zinc sulfate (ZnSO4) and potassium sulfide (K2S) form a Potassium Sulfide And Zinc Bromide Precipitate Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Two important uses of precipitation reactions are to isolate metals. Potassium Sulfide And Zinc Bromide Precipitate.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Potassium Sulfide And Zinc Bromide Precipitate A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Add a few drops of sodium hydroxide solution. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Enter an equation of an ionic chemical equation and. Potassium Sulfide And Zinc Bromide Precipitate.
From www.rpicorp.com
E718005.0 Ethidium Bromide, Powder, 5 Grams Potassium Sulfide And Zinc Bromide Precipitate Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Add a few drops. Potassium Sulfide And Zinc Bromide Precipitate.
From www.drsebiscellfood.com
Bromide Plus Powder Dr. Sebi's Cell Food Potassium Sulfide And Zinc Bromide Precipitate Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover. Potassium Sulfide And Zinc Bromide Precipitate.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Potassium Sulfide And Zinc Bromide Precipitate Enter an equation of an ionic chemical equation and press the balance button. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00. Potassium Sulfide And Zinc Bromide Precipitate.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Potassium Sulfide And Zinc Bromide Precipitate Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Place about 5cm 3 of the solution into a test tube. How. Potassium Sulfide And Zinc Bromide Precipitate.
From www.youtube.com
Does sodium sulfide (Na2S) and zinc nitrate (Zn(NO3)2 precipitate Potassium Sulfide And Zinc Bromide Precipitate If a reaction occurs and a. Place about 5cm 3 of the solution into a test tube. Enter an equation of an ionic chemical equation and press the balance button. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing. Potassium Sulfide And Zinc Bromide Precipitate.
From www.alamy.com
Gel electrophoresis ethidium bromide hires stock photography and Potassium Sulfide And Zinc Bromide Precipitate Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. If a reaction occurs and a. Dissolve a small quantity of the substance in water. How many grams of zinc(ii) nitrate and sodium sulfide. Add a few drops of sodium hydroxide solution. The precipitate is then removed by filtration and the water is brought back to a. Potassium Sulfide And Zinc Bromide Precipitate.
From www.indiamart.com
Sodium Bromide 45, Bromide salt of sodium, NaBr, 7647156, Sedoneural Potassium Sulfide And Zinc Bromide Precipitate Dissolve a small quantity of the substance in water. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Enter an equation of an ionic. Potassium Sulfide And Zinc Bromide Precipitate.
From us.metoree.com
41 Ethyl Bromide Manufacturers in 2024 Metoree Potassium Sulfide And Zinc Bromide Precipitate How many grams of zinc(ii) nitrate and sodium sulfide. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. If a reaction occurs and a. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Two important uses. Potassium Sulfide And Zinc Bromide Precipitate.
From www.indiamart.com
BioTech Grade Powder Sodium Bromide for Petroleum Industry, for Potassium Sulfide And Zinc Bromide Precipitate If a reaction occurs and a. How many grams of zinc(ii) nitrate and sodium sulfide. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Enter an equation of an ionic chemical equation and press the balance button. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds.. Potassium Sulfide And Zinc Bromide Precipitate.
From www.dreamstime.com
Potassium Bromide Chemical Formula on Waterdrop Background Stock Potassium Sulfide And Zinc Bromide Precipitate Dissolve a small quantity of the substance in water. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. When two aqueous solutions of ionic compounds are. Potassium Sulfide And Zinc Bromide Precipitate.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector Potassium Sulfide And Zinc Bromide Precipitate Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. If a reaction occurs and a. How many grams of zinc(ii) nitrate. Potassium Sulfide And Zinc Bromide Precipitate.
From sielc.com
Vinyl bromide SIELC Technologies Potassium Sulfide And Zinc Bromide Precipitate How many grams of zinc(ii) nitrate and sodium sulfide. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Enter an equation of an ionic chemical equation and press the balance button. When. Potassium Sulfide And Zinc Bromide Precipitate.
From www.thistlescientific.co.uk
Ethidium Bromide solution 10mg/ml Thistle Scientific Potassium Sulfide And Zinc Bromide Precipitate How many grams of zinc(ii) nitrate and sodium sulfide. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Place about 5cm 3 of the solution into a test tube. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds.. Potassium Sulfide And Zinc Bromide Precipitate.
From www.numerade.com
SOLVED 'Does a empirical formula of precipitate precipitate form when Potassium Sulfide And Zinc Bromide Precipitate Enter an equation of an ionic chemical equation and press the balance button. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. How many grams of. Potassium Sulfide And Zinc Bromide Precipitate.
From www.ceramic-glazes.com
Potassium bromide Potassium Sulfide And Zinc Bromide Precipitate Dissolve a small quantity of the substance in water. Add a few drops of sodium hydroxide solution. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to. Potassium Sulfide And Zinc Bromide Precipitate.