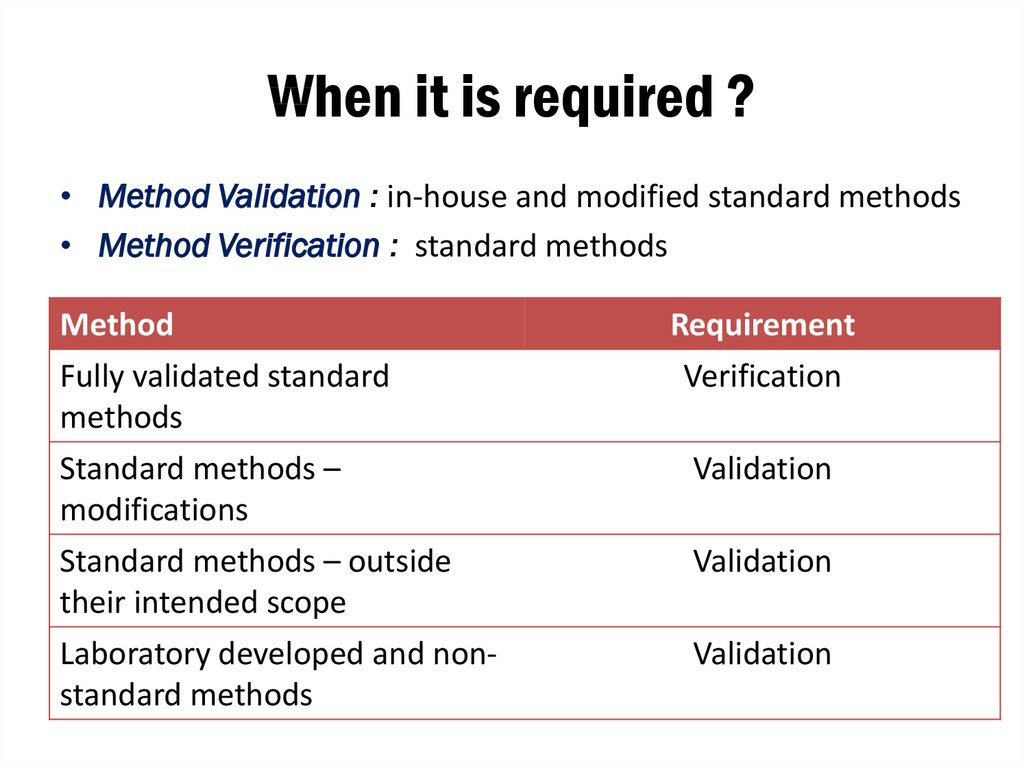
Validation Of Laboratory Tests And Methods . laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. this review summarizes the current literature on the topic, focusing on the requirements for method validations,.
from exofltpju.blob.core.windows.net
the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. this review summarizes the current literature on the topic, focusing on the requirements for method validations,. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument.
What Is Test Method Validation at Karen Coulson blog
Validation Of Laboratory Tests And Methods method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. this review summarizes the current literature on the topic, focusing on the requirements for method validations,. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument.
From www.euformatics.com
Validation of NGS clinical tests Euformatics Validation Of Laboratory Tests And Methods this section of the verification and validation toolkit provides a checklist that walks users through test verification or. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. this review summarizes the current literature on the topic, focusing on the requirements for method validations,. laboratories are. Validation Of Laboratory Tests And Methods.
From exofltpju.blob.core.windows.net
What Is Test Method Validation at Karen Coulson blog Validation Of Laboratory Tests And Methods method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. the purposes of this article. Validation Of Laboratory Tests And Methods.
From www.sofpromed.com
An Introduction to Analytical Method Development and Validation in Validation Of Laboratory Tests And Methods laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. method validation. Validation Of Laboratory Tests And Methods.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Validation Of Laboratory Tests And Methods the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. this review summarizes the current. Validation Of Laboratory Tests And Methods.
From www.researchgate.net
selection for clinical laboratory test method validation in Validation Of Laboratory Tests And Methods laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. this review summarizes the current literature on the topic, focusing on the requirements for method validations,. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. method validation under iso. Validation Of Laboratory Tests And Methods.
From mycoscience.com
Laboratory testing Archives MycoScience Validation Of Laboratory Tests And Methods method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. this review summarizes the current literature on the topic, focusing on the requirements for method validations,. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. the purposes. Validation Of Laboratory Tests And Methods.
From www.researchgate.net
Procedures for Validation of Diagnostic Methods in Clinical Laboratory Validation Of Laboratory Tests And Methods laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. the purposes of this article. Validation Of Laboratory Tests And Methods.
From rjqualityconsulting.com
ISO 17025 Method Validation Ensuring Laboratory Competence and Validation Of Laboratory Tests And Methods this section of the verification and validation toolkit provides a checklist that walks users through test verification or. method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. the purposes. Validation Of Laboratory Tests And Methods.
From gmpinsiders.com
Performance Characteristics In Analytical Method Validation Validation Of Laboratory Tests And Methods method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. the. Validation Of Laboratory Tests And Methods.
From present5.com
Validation of Analytical Methods Hua YIN Outline Validation Of Laboratory Tests And Methods this review summarizes the current literature on the topic, focusing on the requirements for method validations,. method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. . Validation Of Laboratory Tests And Methods.
From mungfali.com
Test Method Validation Validation Of Laboratory Tests And Methods laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. method validation. Validation Of Laboratory Tests And Methods.
From www.researchgate.net
(PDF) Validation of Clinical Laboratory Results Discussion of Validation Of Laboratory Tests And Methods this review summarizes the current literature on the topic, focusing on the requirements for method validations,. method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. laboratories are. Validation Of Laboratory Tests And Methods.
From www.researchgate.net
V3 in practice The verification, analytical validation, and clinical Validation Of Laboratory Tests And Methods this review summarizes the current literature on the topic, focusing on the requirements for method validations,. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. method validation under iso 17025 is a critical procedure. Validation Of Laboratory Tests And Methods.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Validation Of Laboratory Tests And Methods this review summarizes the current literature on the topic, focusing on the requirements for method validations,. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. the purposes of this article. Validation Of Laboratory Tests And Methods.
From www.labcompare.com
Guide to Method Validation of Test Procedures Validation Of Laboratory Tests And Methods laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. the purposes of this article. Validation Of Laboratory Tests And Methods.
From siglaboratory.com
Trial & Method Validation SIG LABORATORY Validation Of Laboratory Tests And Methods this review summarizes the current literature on the topic, focusing on the requirements for method validations,. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. method validation. Validation Of Laboratory Tests And Methods.
From www.researchsop.com
Quality Manual Section 5.4 How Test Method and Method Validation Validation Of Laboratory Tests And Methods method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. the metrology. Validation Of Laboratory Tests And Methods.
From www.researchgate.net
(PDF) Laboratory test method validation Validation Of Laboratory Tests And Methods this section of the verification and validation toolkit provides a checklist that walks users through test verification or. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. the purposes of this article are to present a brief introduction to the concept of test method validation, to. Validation Of Laboratory Tests And Methods.
From www.researchgate.net
(PDF) On the Statistical Testing Methods for Single Laboratory Validation Of Laboratory Tests And Methods the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. this review summarizes the current literature on the topic, focusing on the requirements for method validations,. the metrology. Validation Of Laboratory Tests And Methods.
From cptclabs.com
Reliable Method Validation FDA Compliant CPT Labs Validation Of Laboratory Tests And Methods the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. this review summarizes the current literature on the topic, focusing on the requirements for method validations,. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. laboratories are. Validation Of Laboratory Tests And Methods.
From biotechserv601227982.wordpress.com
The Importance of Lab Equipment Validation Biotechnical Services Validation Of Laboratory Tests And Methods this review summarizes the current literature on the topic, focusing on the requirements for method validations,. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. the purposes of this article are. Validation Of Laboratory Tests And Methods.
From www.prolisphere.com
Why is validation important for a laboratory analyzer Prolis Validation Of Laboratory Tests And Methods the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards. Validation Of Laboratory Tests And Methods.
From mycelx.com
Testing and Validation Lab Validation Of Laboratory Tests And Methods this section of the verification and validation toolkit provides a checklist that walks users through test verification or. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. the purposes of this article are to present a brief introduction to the concept of test method validation, to. Validation Of Laboratory Tests And Methods.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Validation Of Laboratory Tests And Methods this section of the verification and validation toolkit provides a checklist that walks users through test verification or. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. laboratories are required. Validation Of Laboratory Tests And Methods.
From www.sentrakalibrasiindustri.com
Kapan Melakukan Verifikasi dan Validasi Metode Analisis? Validation Of Laboratory Tests And Methods the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. this review summarizes. Validation Of Laboratory Tests And Methods.
From www.researchgate.net
(PDF) Guidelines for the singlelaboratory validation of qualitative Validation Of Laboratory Tests And Methods method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. . Validation Of Laboratory Tests And Methods.
From www.pharmamirror.com
Bioanalytical Method Validation and its Current Updates Pharma Mirror Validation Of Laboratory Tests And Methods laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. . Validation Of Laboratory Tests And Methods.
From www.degruyter.com
Validation and verification of examination procedures in medical Validation Of Laboratory Tests And Methods the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. this review summarizes the current literature on the topic, focusing on the requirements for method validations,. laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. method validation under iso. Validation Of Laboratory Tests And Methods.
From dxolpaaop.blob.core.windows.net
Verification Validation Test at Lisa Perry blog Validation Of Laboratory Tests And Methods the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. laboratories are required. Validation Of Laboratory Tests And Methods.
From www.slideserve.com
PPT A StepbyStep Guide for Method Validation PowerPoint Validation Of Laboratory Tests And Methods the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. the purposes of this article are to present a brief introduction to the concept of test method. Validation Of Laboratory Tests And Methods.
From pharmanotes.org
Method Validation in Regulated Lab Pharmanotes Validation Of Laboratory Tests And Methods laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. . Validation Of Laboratory Tests And Methods.
From studylib.net
Laboratory Method Validation For any method that will be used for Validation Of Laboratory Tests And Methods method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. this review summarizes the current literature on the topic, focusing on the requirements for method validations,. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. the purposes. Validation Of Laboratory Tests And Methods.
From www.researchgate.net
(PDF) Validation of analytical methods and laboratory procedures for Validation Of Laboratory Tests And Methods laboratories are required to perform analytical validation or verification of each nonwaived test, method, or instrument. the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. the purposes. Validation Of Laboratory Tests And Methods.
From www.researchgate.net
(PDF) Validation of Clinical Laboratory Results Discussion of Validation Of Laboratory Tests And Methods the purposes of this article are to present a brief introduction to the concept of test method validation, to describe. this review summarizes the current literature on the topic, focusing on the requirements for method validations,. method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. . Validation Of Laboratory Tests And Methods.
From dxofqtloz.blob.core.windows.net
Test Method Validation Vs Gage R&R at Jeffrey Rutherford blog Validation Of Laboratory Tests And Methods method validation under iso 17025 is a critical procedure that ensures your laboratory’s testing methods meet the required standards for. this review summarizes the current literature on the topic, focusing on the requirements for method validations,. this section of the verification and validation toolkit provides a checklist that walks users through test verification or. the metrology. Validation Of Laboratory Tests And Methods.