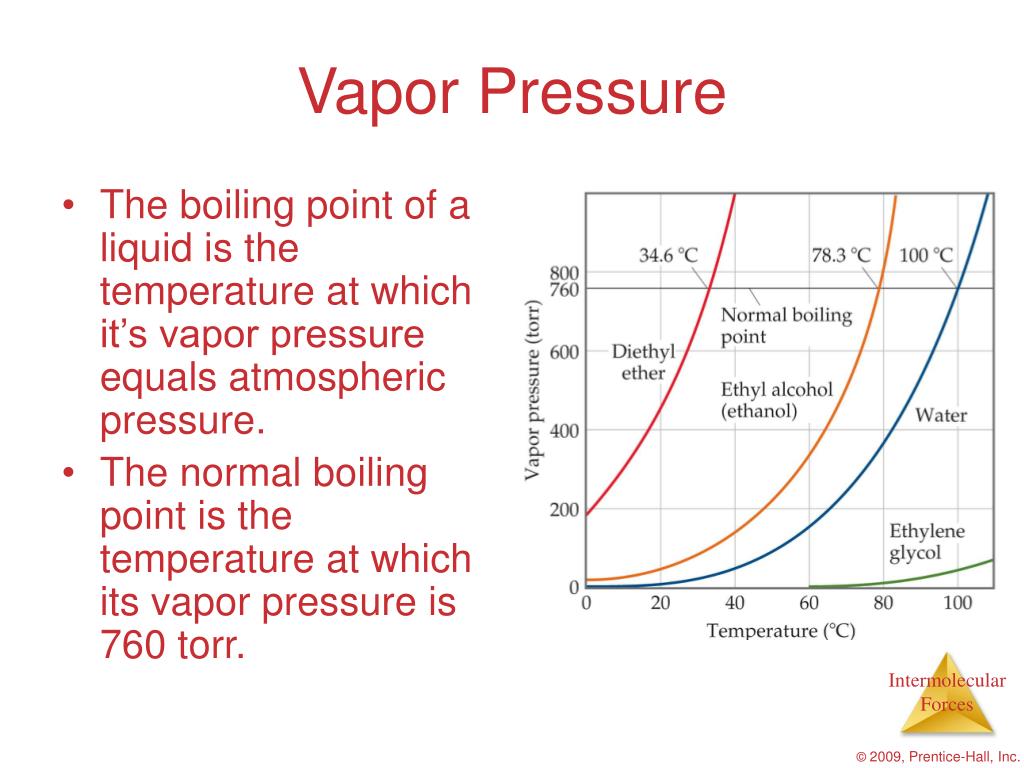
Evaporation Effect Intermolecular Forces . The stronger these forces, the lower. Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. The web page explains the order of. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). How do intermolecular forces affect evaporation? Evaporation of a volatile liquid is an endothermic process that results in a temperature decrease. The magnitude of temperature decrease is related to. In order for a liquid molecule to escape into the gas state, the molecule must have. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules.
from www.slideserve.com
Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. The stronger these forces, the lower. The web page explains the order of. Evaporation of a volatile liquid is an endothermic process that results in a temperature decrease. Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. In order for a liquid molecule to escape into the gas state, the molecule must have. The magnitude of temperature decrease is related to. How do intermolecular forces affect evaporation?
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247
Evaporation Effect Intermolecular Forces In order for a liquid molecule to escape into the gas state, the molecule must have. The web page explains the order of. The stronger these forces, the lower. The magnitude of temperature decrease is related to. Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Evaporation of a volatile liquid is an endothermic process that results in a temperature decrease. In order for a liquid molecule to escape into the gas state, the molecule must have. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. How do intermolecular forces affect evaporation?
From www.youtube.com
Evaporation and Intermolecular Attractions YouTube Evaporation Effect Intermolecular Forces Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. How do intermolecular forces affect evaporation? The web page explains the order. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 Evaporation Effect Intermolecular Forces A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. The stronger these forces, the lower. The web page explains the order of. In order for a liquid molecule to escape into the gas state, the molecule must have. Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily.. Evaporation Effect Intermolecular Forces.
From studylib.net
Intermolecular Forces Evaporation Effect Intermolecular Forces The magnitude of temperature decrease is related to. In order for a liquid molecule to escape into the gas state, the molecule must have. How do intermolecular forces affect evaporation? The stronger these forces, the lower. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). A liquid’s vapor pressure is directly related. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Chapter 12 Intermolecular Forces and Liquids PowerPoint Presentation ID856300 Evaporation Effect Intermolecular Forces Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. The stronger these forces, the lower. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). In order for a liquid molecule to escape into the gas state, the molecule must have. Evaporation of a volatile liquid. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 Evaporation Effect Intermolecular Forces The magnitude of temperature decrease is related to. Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). The stronger these forces, the lower. How do intermolecular forces affect evaporation? The web page explains the order of. A. Evaporation Effect Intermolecular Forces.
From slideplayer.com
Chapter Intermolecular Forces ppt download Evaporation Effect Intermolecular Forces How do intermolecular forces affect evaporation? Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). In order for a liquid molecule to escape into the gas state, the molecule must have. Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. The magnitude of temperature decrease is. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces of Attraction PowerPoint Presentation, free download ID260295 Evaporation Effect Intermolecular Forces The magnitude of temperature decrease is related to. Evaporation of a volatile liquid is an endothermic process that results in a temperature decrease. The web page explains the order of. How do intermolecular forces affect evaporation? In order for a liquid molecule to escape into the gas state, the molecule must have. The stronger these forces, the lower. Learn how. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 Evaporation Effect Intermolecular Forces The stronger these forces, the lower. The magnitude of temperature decrease is related to. The web page explains the order of. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). In order for a liquid molecule to. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT 14.1 Molecular Substances Intermolecular Forces PowerPoint Presentation ID2755968 Evaporation Effect Intermolecular Forces Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. Learn how to predict the relative boiling points of organic compounds based on their. Evaporation Effect Intermolecular Forces.
From www.youtube.com
Intermolecular Forces, Surface Tension, & Evaporation YouTube Evaporation Effect Intermolecular Forces The stronger these forces, the lower. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. The magnitude of temperature decrease is. Evaporation Effect Intermolecular Forces.
From www.studocu.com
Intermolecular+Forces Evaporation, Temperature, and Intermolecular Forces Objectives To Evaporation Effect Intermolecular Forces In order for a liquid molecule to escape into the gas state, the molecule must have. The web page explains the order of. The magnitude of temperature decrease is related to. Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. Learn how to predict the relative boiling points of organic compounds based on their. Evaporation Effect Intermolecular Forces.
From www.youtube.com
12 Nust Entrance Test Rate of Evaporation, Intermolecular Forces (Chemistry) YouTube Evaporation Effect Intermolecular Forces The web page explains the order of. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. The magnitude of temperature decrease is related to. Evaporation of a volatile liquid is an endothermic process that results in a temperature decrease. Learn how to predict the relative boiling points of organic compounds based on their intermolecular. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 Evaporation Effect Intermolecular Forces The stronger these forces, the lower. In order for a liquid molecule to escape into the gas state, the molecule must have. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. How do intermolecular forces affect evaporation? The web page explains the order of. Liquids with weak intermolecular attractive forces have low heats of. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 Evaporation Effect Intermolecular Forces Evaporation of a volatile liquid is an endothermic process that results in a temperature decrease. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). The web page explains the order of. The magnitude of temperature decrease is related to. In order for a liquid molecule to escape into the gas state, the. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids, Solids, and Intermolecular Forces PowerPoint Presentation ID6376789 Evaporation Effect Intermolecular Forces The magnitude of temperature decrease is related to. In order for a liquid molecule to escape into the gas state, the molecule must have. How do intermolecular forces affect evaporation? A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure.. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint Presentation ID5950788 Evaporation Effect Intermolecular Forces The magnitude of temperature decrease is related to. Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. The web page explains the order of. In order for a liquid molecule to escape into the gas state, the molecule must have. The stronger these forces, the lower. Learn how intermolecular forces affect the rate. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 Evaporation Effect Intermolecular Forces The magnitude of temperature decrease is related to. Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. The web page explains the order. Evaporation Effect Intermolecular Forces.
From slideplayer.com
Intermolecular forces ppt download Evaporation Effect Intermolecular Forces Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. In order for a liquid molecule to escape into the gas state,. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 Evaporation Effect Intermolecular Forces Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). The magnitude of temperature decrease is related to. The web page explains the order of. In order for a liquid molecule to escape into the gas state,. Evaporation Effect Intermolecular Forces.
From www.youtube.com
Evaporation and Intermolecular Forces YouTube Evaporation Effect Intermolecular Forces Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). In order for a liquid molecule to escape into the gas state, the molecule must have. Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. The web page explains the order of. The magnitude of temperature decrease. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID2286693 Evaporation Effect Intermolecular Forces A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. How do intermolecular forces affect evaporation? Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. In order for a liquid molecule. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID9166403 Evaporation Effect Intermolecular Forces How do intermolecular forces affect evaporation? A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. The web page explains the order of. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). The stronger these forces, the lower. Liquids with weak intermolecular attractive forces have low heats. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces of Attraction PowerPoint Presentation, free download ID260295 Evaporation Effect Intermolecular Forces Evaporation of a volatile liquid is an endothermic process that results in a temperature decrease. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. In order for a liquid molecule to escape into the gas state, the. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID2286693 Evaporation Effect Intermolecular Forces The web page explains the order of. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). In order for a liquid molecule to escape into the gas state, the molecule must have. The magnitude of temperature decrease is related to. The stronger these forces, the lower. How do intermolecular forces affect evaporation?. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 Evaporation Effect Intermolecular Forces A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. The magnitude of temperature decrease is related to. The stronger these forces, the lower. In order for a liquid molecule to escape into the gas state, the molecule must have. Evaporation of a volatile liquid is an endothermic process that results in a temperature decrease.. Evaporation Effect Intermolecular Forces.
From chemistrytalk.org
Influence of Intermolecular Forces ChemTalk Evaporation Effect Intermolecular Forces In order for a liquid molecule to escape into the gas state, the molecule must have. A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. How do intermolecular forces affect evaporation? The web page explains the order of. Evaporation. Evaporation Effect Intermolecular Forces.
From www.studocu.com
01 Evaporation and Intermolecular Forces Evaporation and Intermolecular Forces Essential Evaporation Effect Intermolecular Forces A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. How do intermolecular forces affect evaporation? Evaporation of a volatile liquid is an endothermic process that results in a temperature decrease. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). The magnitude of temperature decrease is related. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 Evaporation Effect Intermolecular Forces The magnitude of temperature decrease is related to. The stronger these forces, the lower. Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. The web page explains the order of. In order for a liquid molecule to escape into the gas state, the molecule must have. How do intermolecular forces affect evaporation? A. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 Evaporation Effect Intermolecular Forces Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). How do intermolecular forces affect evaporation? Evaporation of a volatile liquid is an endothermic process that results in a temperature decrease. Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. The magnitude of temperature decrease is related. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 Evaporation Effect Intermolecular Forces A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. How do intermolecular forces affect evaporation? Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. The stronger these forces, the lower.. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Presentation ID819247 Evaporation Effect Intermolecular Forces The stronger these forces, the lower. Learn how to predict the relative boiling points of organic compounds based on their intermolecular forces (imfs). Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. The web page explains the order of. The magnitude of temperature decrease is related to. Learn how intermolecular forces affect the. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Vaporization PowerPoint Presentation, free download ID3207099 Evaporation Effect Intermolecular Forces Liquids with weak intermolecular attractive forces have low heats of vaporization and are volatile—they evaporate easily. The web page explains the order of. Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. The stronger these forces, the lower. In order for a liquid molecule to escape into the gas state, the molecule must have.. Evaporation Effect Intermolecular Forces.
From www.youtube.com
Intermolecular Forces Trends Melting & Boiling Point, Viscosity, Surface Tension, Vapor Evaporation Effect Intermolecular Forces How do intermolecular forces affect evaporation? A liquid’s vapor pressure is directly related to the intermolecular forces present between its molecules. Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. The stronger these forces, the lower. In order for a liquid molecule to escape into the gas state, the molecule must have. The magnitude. Evaporation Effect Intermolecular Forces.
From www.studocu.com
Evaporation Intermolecular Forces SCH4U October 24th, 2017 The Effect Intermolecular Forces Evaporation Effect Intermolecular Forces In order for a liquid molecule to escape into the gas state, the molecule must have. The stronger these forces, the lower. Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. How do intermolecular forces affect evaporation? Evaporation of a volatile liquid is an endothermic process that results in a temperature decrease. The magnitude. Evaporation Effect Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Presentation ID3374064 Evaporation Effect Intermolecular Forces The stronger these forces, the lower. Evaporation of a volatile liquid is an endothermic process that results in a temperature decrease. In order for a liquid molecule to escape into the gas state, the molecule must have. Learn how intermolecular forces affect the rate of evaporation of a liquid and vapor pressure. A liquid’s vapor pressure is directly related to. Evaporation Effect Intermolecular Forces.