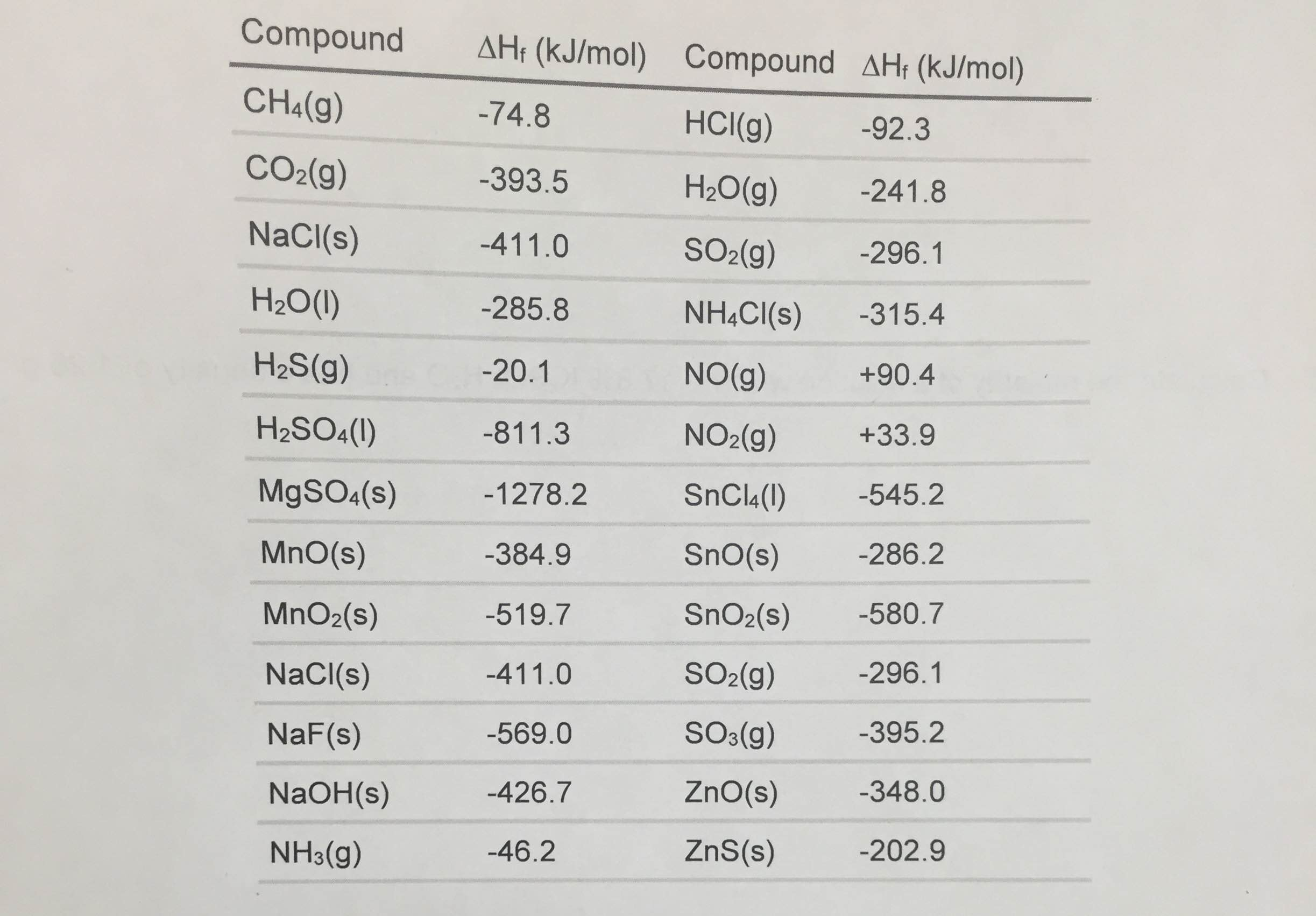
Standard Enthalpy Of Formation Potassium Fluoride . The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution), the pressure. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,.
from mungfali.com
136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution), the pressure.
Enthalpies Of Formation Chart
Standard Enthalpy Of Formation Potassium Fluoride 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution), the pressure. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the.
From www.numerade.com
SOLVED The diagram rcprcscnts the BornHaber cycle for the formation of crystalline potassium Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Of Formation Potassium Fluoride.
From www.chegg.com
Solved 8) a) Define "standard enthalpy change of formation." Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard. Standard Enthalpy Of Formation Potassium Fluoride.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Potassium Fluoride The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution), the pressure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables. Standard Enthalpy Of Formation Potassium Fluoride.
From schoolworkhelper.net
Standard Enthalpies of Formation Online Homework Help SchoolWorkHelper Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Of Formation Potassium Fluoride.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The magnitude of δ h for a reaction depends on the physical. Standard Enthalpy Of Formation Potassium Fluoride.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Potassium Fluoride The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution), the pressure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy. Standard Enthalpy Of Formation Potassium Fluoride.
From www.w3schools.blog
Standard enthalpy of ionization W3schools Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy change of formation is the sum of the heats of. Standard Enthalpy Of Formation Potassium Fluoride.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Change YouTube Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat. Standard Enthalpy Of Formation Potassium Fluoride.
From askfilo.com
Determine the standard enthalpy change of solution of potassium phosphate.. Standard Enthalpy Of Formation Potassium Fluoride 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution), the pressure. The standard enthalpy of formation is a measure of the energy released. Standard Enthalpy Of Formation Potassium Fluoride.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Appendix I to calculate the Standard Enthalpy Of Formation Potassium Fluoride 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. 136 rows standard enthalpy change of formation (data table) these tables include. Standard Enthalpy Of Formation Potassium Fluoride.
From www.studeersnel.nl
Rstandard enthalpy of formation Table useful Standard Enthalpy of Formation* for Various Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Of Formation Potassium Fluoride.
From slideplayer.com
STANDARD MOLAR ENTHALPY OF FORMATION ppt download Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation. Standard Enthalpy Of Formation Potassium Fluoride.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5773812 Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Of Formation Potassium Fluoride.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Potassium Fluoride 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 136 rows standard enthalpy change of formation (data table) these tables include heat. Standard Enthalpy Of Formation Potassium Fluoride.
From brainly.com
Calculate the enthalpies of formation, ΔHf∘, of the group 1 fluoride compounds from their Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat. Standard Enthalpy Of Formation Potassium Fluoride.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies of Formation YouTube Standard Enthalpy Of Formation Potassium Fluoride The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution), the pressure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy. Standard Enthalpy Of Formation Potassium Fluoride.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The magnitude of δ h for a reaction depends on the. Standard Enthalpy Of Formation Potassium Fluoride.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The magnitude of δ h for a reaction depends on the physical states. Standard Enthalpy Of Formation Potassium Fluoride.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution), the pressure. 193 rows in chemistry and thermodynamics, the standard enthalpy. Standard Enthalpy Of Formation Potassium Fluoride.
From www.numerade.com
the diagram below is the bom huber cycle for the formation of crystalline potassium fluoride ktg Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation. Standard Enthalpy Of Formation Potassium Fluoride.
From slideplayer.com
Standard Enthalpies of Formation ppt download Standard Enthalpy Of Formation Potassium Fluoride The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution), the pressure. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. The standard enthalpy of formation is a measure of. Standard Enthalpy Of Formation Potassium Fluoride.
From solvedlib.com
Calculate the lattice energy of potassium fluoride, K… SolvedLib Standard Enthalpy Of Formation Potassium Fluoride The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution), the pressure. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Standard Enthalpy Of Formation Potassium Fluoride.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Potassium Fluoride 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard. Standard Enthalpy Of Formation Potassium Fluoride.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Potassium Fluoride 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy change of formation is the sum of the heats of formation of. Standard Enthalpy Of Formation Potassium Fluoride.
From www.chemistrylearner.com
Potassium Fluoride Facts, Formula, Properties, Uses, Safety Data Standard Enthalpy Of Formation Potassium Fluoride The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution), the pressure. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. 136 rows standard enthalpy change of formation (data table). Standard Enthalpy Of Formation Potassium Fluoride.
From joilylugg.blob.core.windows.net
Standard Enthalpy Of Formation Def at Sandra Leonard blog Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The magnitude of δ h for a reaction depends on the physical states of. Standard Enthalpy Of Formation Potassium Fluoride.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free download ID4097208 Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include. Standard Enthalpy Of Formation Potassium Fluoride.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation Potassium Fluoride 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution), the pressure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Standard Enthalpy Of Formation Potassium Fluoride.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all answers to three sig figs Standard Enthalpy Of Formation Potassium Fluoride 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. The standard enthalpy of formation is a measure of the energy released. Standard Enthalpy Of Formation Potassium Fluoride.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Potassium Fluoride 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. The magnitude of δ h for a reaction depends on the physical states. Standard Enthalpy Of Formation Potassium Fluoride.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Chemistry 2e 5.3 YouTube Standard Enthalpy Of Formation Potassium Fluoride 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. The standard enthalpy of formation is a measure of the energy released. Standard Enthalpy Of Formation Potassium Fluoride.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free download ID4820880 Standard Enthalpy Of Formation Potassium Fluoride 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Of Formation Potassium Fluoride.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work... Download Table Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released. Standard Enthalpy Of Formation Potassium Fluoride.
From www.chegg.com
Solved The diagram below is the BornHaber cycle for the Standard Enthalpy Of Formation Potassium Fluoride 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or solution), the pressure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Standard Enthalpy Of Formation Potassium Fluoride.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation Potassium Fluoride The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy change of formation is the sum of the heats of. Standard Enthalpy Of Formation Potassium Fluoride.