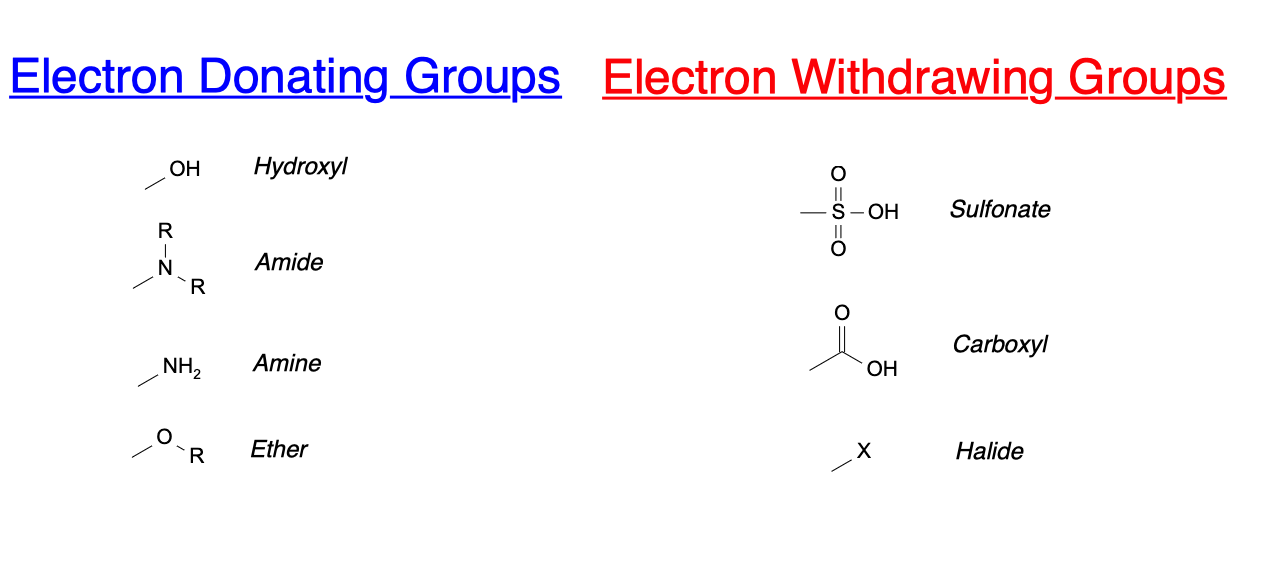
Chlorine Electron Donating Or Withdrawing . Because f pulls electrons toward itself, and positively polarizes the c to which it is. If a substituent increases the rate of reaction. For electrophilic aromatic substitution (eas) reactions,. When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. Substituent groups can be electron withdrawing or electron donating. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Groups that can donate electron density to the ring make eas reactions faster.
from orgosolver.com
Groups that can donate electron density to the ring make eas reactions faster. If a substituent increases the rate of reaction. Because f pulls electrons toward itself, and positively polarizes the c to which it is. When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. For electrophilic aromatic substitution (eas) reactions,. Substituent groups can be electron withdrawing or electron donating. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity.
OrgoSolver
Chlorine Electron Donating Or Withdrawing Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. For electrophilic aromatic substitution (eas) reactions,. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. If a substituent increases the rate of reaction. Groups that can donate electron density to the ring make eas reactions faster. When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Substituent groups can be electron withdrawing or electron donating. Because f pulls electrons toward itself, and positively polarizes the c to which it is.
From www.youtube.com
Although chlorine is an electronwithdrawing group yet it is ortho Chlorine Electron Donating Or Withdrawing Substituent groups can be electron withdrawing or electron donating. Because f pulls electrons toward itself, and positively polarizes the c to which it is. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Groups that can donate electron density to the ring make eas reactions faster. For electrophilic aromatic substitution (eas) reactions,. Halogens are. Chlorine Electron Donating Or Withdrawing.
From www.researchgate.net
Effect of electron withdrawing or electron donating groups on reduction Chlorine Electron Donating Or Withdrawing Because f pulls electrons toward itself, and positively polarizes the c to which it is. For electrophilic aromatic substitution (eas) reactions,. If a substituent increases the rate of reaction. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. When functional. Chlorine Electron Donating Or Withdrawing.
From www.researchgate.net
Selected electron‐donating and electron‐withdrawing groups with Chlorine Electron Donating Or Withdrawing Because f pulls electrons toward itself, and positively polarizes the c to which it is. For electrophilic aromatic substitution (eas) reactions,. Groups that can donate electron density to the ring make eas reactions faster. If a substituent increases the rate of reaction. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. When functional groups. Chlorine Electron Donating Or Withdrawing.
From orgosolver.com
OrgoSolver Chlorine Electron Donating Or Withdrawing Substituent groups can be electron withdrawing or electron donating. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. If a substituent increases the rate of reaction. Because f pulls electrons toward itself, and positively polarizes the c to which it is. For electrophilic aromatic substitution (eas) reactions,. Halogens are considered deactivating groups due to. Chlorine Electron Donating Or Withdrawing.
From www.pinterest.com
صورة ذات صلة Organic chemistry, Organic chem, Chemistry Chlorine Electron Donating Or Withdrawing Substituent groups can be electron withdrawing or electron donating. When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. Because f pulls electrons toward itself, and positively polarizes the c to which it is. If a substituent increases the rate of reaction. Halogens are considered deactivating groups due to the inductive. Chlorine Electron Donating Or Withdrawing.
From www.researchgate.net
Electron donating ability comparison among heteroatoms of O, N and S Chlorine Electron Donating Or Withdrawing Substituent groups can be electron withdrawing or electron donating. Groups that can donate electron density to the ring make eas reactions faster. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. Because f pulls electrons toward itself, and positively polarizes. Chlorine Electron Donating Or Withdrawing.
From www.youtube.com
Although chlorine is an electron withdrawing group, yet it is ortho Chlorine Electron Donating Or Withdrawing Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Groups that can donate electron density to the ring make eas reactions faster. If a substituent increases the rate of reaction. When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. Because f pulls electrons toward. Chlorine Electron Donating Or Withdrawing.
From www.numerade.com
SOLVED Classify each substituent as electron donating or electron Chlorine Electron Donating Or Withdrawing When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. Groups that can donate electron density to the ring make eas reactions faster. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. For electrophilic aromatic substitution (eas) reactions,. If a substituent increases the rate of. Chlorine Electron Donating Or Withdrawing.
From www.linstitute.net
CIE A Level Chemistry复习笔记7.2.4 Directing Effects of Substituents on Chlorine Electron Donating Or Withdrawing For electrophilic aromatic substitution (eas) reactions,. Because f pulls electrons toward itself, and positively polarizes the c to which it is. If a substituent increases the rate of reaction. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. Groups that can donate electron density to the ring make eas reactions faster. Substituent groups can be. Chlorine Electron Donating Or Withdrawing.
From www.youtube.com
Electrondonating and Electronwithdrawing Groups YouTube Chlorine Electron Donating Or Withdrawing Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. For electrophilic aromatic substitution (eas) reactions,. Because f pulls electrons toward itself, and positively polarizes the c to which it is. Groups that can donate electron density to the ring make eas reactions faster. Substituent groups can be electron withdrawing or electron donating. Halogens are. Chlorine Electron Donating Or Withdrawing.
From electronic--racket.blogspot.com
How To Identify Electron Withdrawing And Donating Groups Chlorine Electron Donating Or Withdrawing Groups that can donate electron density to the ring make eas reactions faster. If a substituent increases the rate of reaction. Because f pulls electrons toward itself, and positively polarizes the c to which it is. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Halogens are considered deactivating groups due to the inductive. Chlorine Electron Donating Or Withdrawing.
From www.doubtnut.com
Although chlorine is an electronwithdrawing group, yet it is ortho, Chlorine Electron Donating Or Withdrawing For electrophilic aromatic substitution (eas) reactions,. Groups that can donate electron density to the ring make eas reactions faster. If a substituent increases the rate of reaction. When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction. Chlorine Electron Donating Or Withdrawing.
From chemistry.stackexchange.com
organic chemistry Why are halogens ortho para directing even though Chlorine Electron Donating Or Withdrawing Because f pulls electrons toward itself, and positively polarizes the c to which it is. If a substituent increases the rate of reaction. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Groups that can donate electron density to the ring make eas reactions faster. Halogens are considered deactivating groups due to the inductive. Chlorine Electron Donating Or Withdrawing.
From byjus.com
26.how does the attachment of an electron withdrawing group like NO2 in Chlorine Electron Donating Or Withdrawing Groups that can donate electron density to the ring make eas reactions faster. If a substituent increases the rate of reaction. For electrophilic aromatic substitution (eas) reactions,. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. Substituent groups can be electron withdrawing or electron donating. When functional groups like alkyl groups (such as methyl, ethyl,. Chlorine Electron Donating Or Withdrawing.
From byjus.com
35. Effect of electron donating group on acidity of phenol. Explain Chlorine Electron Donating Or Withdrawing Groups that can donate electron density to the ring make eas reactions faster. Substituent groups can be electron withdrawing or electron donating. Because f pulls electrons toward itself, and positively polarizes the c to which it is. If a substituent increases the rate of reaction. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. For. Chlorine Electron Donating Or Withdrawing.
From www.pearson.com
Electron Withdrawing Groups Online Tutor, Practice Problems & Exam Prep Chlorine Electron Donating Or Withdrawing Groups that can donate electron density to the ring make eas reactions faster. For electrophilic aromatic substitution (eas) reactions,. Substituent groups can be electron withdrawing or electron donating. If a substituent increases the rate of reaction. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Because f pulls electrons toward itself, and positively polarizes. Chlorine Electron Donating Or Withdrawing.
From byjus.com
Why does electron withdrawing group increases the acidity and releasing Chlorine Electron Donating Or Withdrawing Because f pulls electrons toward itself, and positively polarizes the c to which it is. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. For electrophilic aromatic substitution (eas) reactions,. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. When functional groups like alkyl groups (such as methyl, ethyl,. Chlorine Electron Donating Or Withdrawing.
From www.youtube.com
30.04 Electrondonating and withdrawing Groups YouTube Chlorine Electron Donating Or Withdrawing Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Groups that can donate electron density to the ring make eas reactions faster. Substituent groups can be electron withdrawing or electron donating. When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. If a substituent increases. Chlorine Electron Donating Or Withdrawing.
From www.slideserve.com
PPT Electrophilic Aromatic Substitution PowerPoint Presentation ID Chlorine Electron Donating Or Withdrawing When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. If a substituent increases the rate of reaction. For electrophilic aromatic substitution (eas) reactions,. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. Substituent groups can be electron withdrawing or electron donating. Recognizing substituents as electron. Chlorine Electron Donating Or Withdrawing.
From byjus.com
47. List of electron withdrawing group and electron donating group and Chlorine Electron Donating Or Withdrawing Because f pulls electrons toward itself, and positively polarizes the c to which it is. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. If a substituent increases the rate of reaction. For electrophilic aromatic substitution (eas) reactions,. Groups that can donate electron density to the ring make eas reactions faster. When functional groups. Chlorine Electron Donating Or Withdrawing.
From www.scribd.com
Electron Withdrawing and Electron Donating Groups Functional Group Ion Chlorine Electron Donating Or Withdrawing Because f pulls electrons toward itself, and positively polarizes the c to which it is. Groups that can donate electron density to the ring make eas reactions faster. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. If a substituent increases the rate of reaction. When functional groups like alkyl groups (such as methyl, ethyl,. Chlorine Electron Donating Or Withdrawing.
From www.numerade.com
SOLVED ElectronWithdrawing Groups in Carbonyls One other thing to Chlorine Electron Donating Or Withdrawing If a substituent increases the rate of reaction. Substituent groups can be electron withdrawing or electron donating. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they. Chlorine Electron Donating Or Withdrawing.
From askfilo.com
10.9 Although chlorine is an electron withdrawing group, yet it is ortho.. Chlorine Electron Donating Or Withdrawing When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. Groups that can donate electron density to the ring make eas reactions faster. For electrophilic aromatic substitution (eas) reactions,. If a substituent increases the rate of reaction. Because f pulls electrons toward itself, and positively polarizes the c to which it. Chlorine Electron Donating Or Withdrawing.
From www.youtube.com
Electron Withdrawing & Donating Groups Polarity YouTube Chlorine Electron Donating Or Withdrawing When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. If a substituent increases the rate of reaction. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. Groups that can donate electron density to the ring make eas reactions faster. Substituent groups can be electron withdrawing. Chlorine Electron Donating Or Withdrawing.
From www.researchgate.net
The effect of electronwithdrawing and electrondonating groups on Chlorine Electron Donating Or Withdrawing Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Groups that can donate electron density to the ring make eas reactions faster. When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. Halogens are considered deactivating groups due to the inductive effects of their overwhelming. Chlorine Electron Donating Or Withdrawing.
From www.youtube.com
Summary of electron.donating and withdrawing groups. Reactivity toward Chlorine Electron Donating Or Withdrawing Groups that can donate electron density to the ring make eas reactions faster. If a substituent increases the rate of reaction. Because f pulls electrons toward itself, and positively polarizes the c to which it is. Substituent groups can be electron withdrawing or electron donating. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. When. Chlorine Electron Donating Or Withdrawing.
From askfilo.com
Exarple 10.9 Although chlorine is an electron withdrawing group, yet it i.. Chlorine Electron Donating Or Withdrawing If a substituent increases the rate of reaction. When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. For electrophilic aromatic substitution (eas) reactions,. Substituent groups can be electron withdrawing or electron donating. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. Recognizing substituents as electron. Chlorine Electron Donating Or Withdrawing.
From brainly.in
although chlorine is electron withdrawing group yet it is Ortho and Chlorine Electron Donating Or Withdrawing If a substituent increases the rate of reaction. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Because f pulls electrons toward itself, and positively polarizes the c to which it is. For electrophilic aromatic substitution (eas) reactions,. Groups that can donate electron density to the ring make eas reactions faster. When functional groups. Chlorine Electron Donating Or Withdrawing.
From www.slideserve.com
PPT Chemistry of Aromatic Compounds PowerPoint Presentation, free Chlorine Electron Donating Or Withdrawing When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. For electrophilic aromatic substitution (eas) reactions,. Groups that can donate electron density to the ring make eas reactions faster. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. If a substituent increases the rate of reaction.. Chlorine Electron Donating Or Withdrawing.
From askfilo.com
Although chlorine is an electron withdrawing group, yet it is ortho, par.. Chlorine Electron Donating Or Withdrawing When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. If a substituent increases the rate of reaction. Groups that can donate electron density to the ring make eas reactions faster. For electrophilic aromatic substitution (eas) reactions,. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity.. Chlorine Electron Donating Or Withdrawing.
From byjus.com
How does adding an electron withdrawing group to buy benzene diazonium Chlorine Electron Donating Or Withdrawing If a substituent increases the rate of reaction. Because f pulls electrons toward itself, and positively polarizes the c to which it is. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. Groups that can donate. Chlorine Electron Donating Or Withdrawing.
From www.toppr.com
Although chlorine is an electron withdrawing group, yet it is ortho Chlorine Electron Donating Or Withdrawing Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Substituent groups can be electron withdrawing or electron donating. Because f pulls electrons toward itself, and positively polarizes the c to which it is. Groups that can donate electron density to the ring make eas reactions faster. For electrophilic aromatic substitution (eas) reactions,. Halogens are. Chlorine Electron Donating Or Withdrawing.
From forums.studentdoctor.net
Resonance Electron Withdrawing or Donating Student Doctor Network Chlorine Electron Donating Or Withdrawing Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. If a substituent increases the rate of reaction. Substituent groups can be. Chlorine Electron Donating Or Withdrawing.
From www.numerade.com
OH Cl D Less acidic; as Cl is an electron donating group. More acidic Chlorine Electron Donating Or Withdrawing When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. Recognizing substituents as electron donating or withdrawing is a useful skill for evaluating reaction mechanisms. Substituent groups can be electron withdrawing or electron donating. Halogens are considered deactivating groups due to the inductive effects of their overwhelming electronegativity. For electrophilic aromatic. Chlorine Electron Donating Or Withdrawing.
From kmsfert.blogspot.com
Electron Withdrawing Groups List / Effects of electron withdrawing Chlorine Electron Donating Or Withdrawing Groups that can donate electron density to the ring make eas reactions faster. If a substituent increases the rate of reaction. When functional groups like alkyl groups (such as methyl, ethyl, etc.) donate electrons they exhibit a positive inductive effect. Because f pulls electrons toward itself, and positively polarizes the c to which it is. Halogens are considered deactivating groups. Chlorine Electron Donating Or Withdrawing.