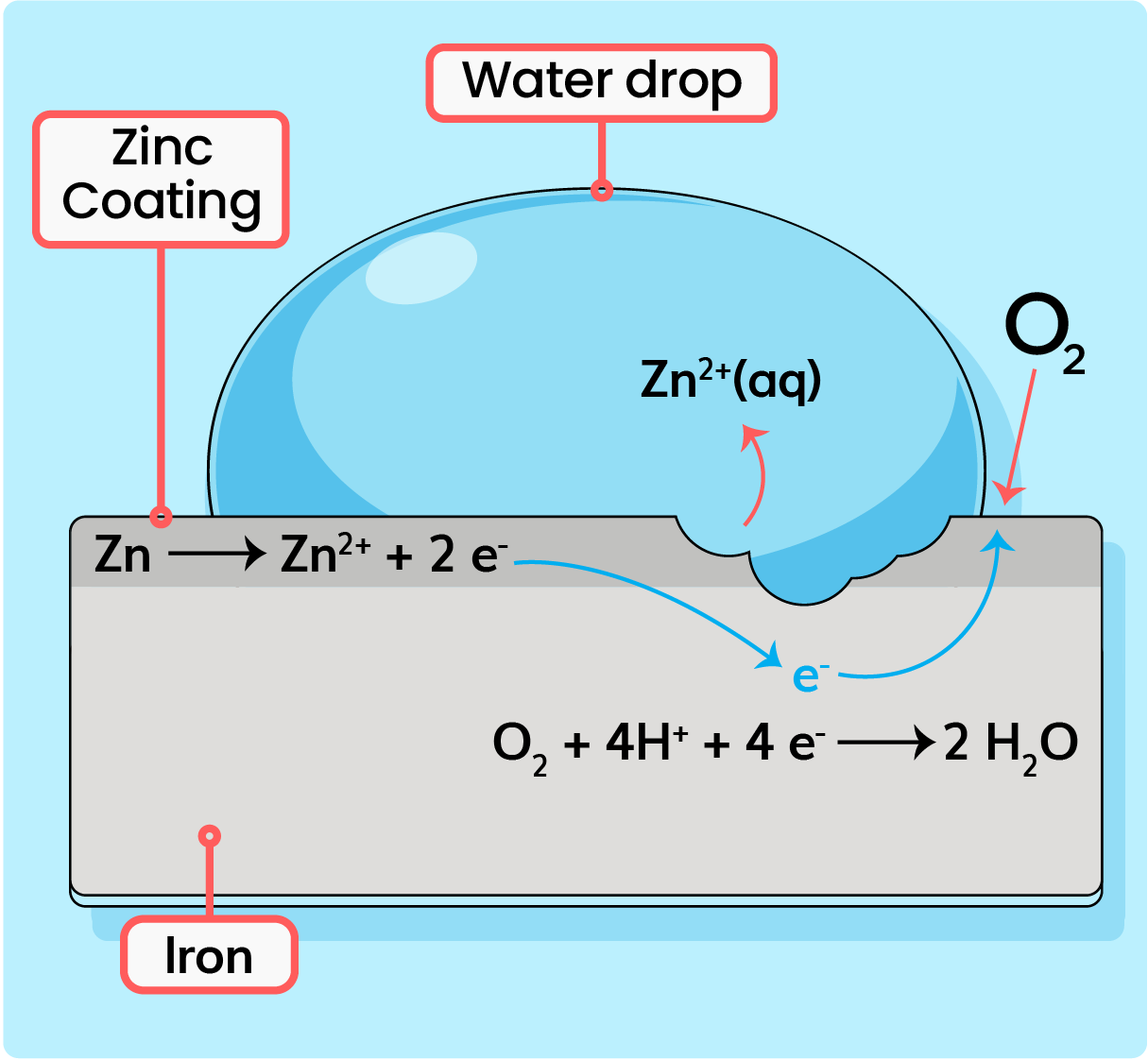
How Does Zinc Stop Rust . Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products. The iron or steel object is coated in a thin layer of zinc. However, this element does not rust like most other metals. When exposed to oxygen and moisture, zinc reacts to create zinc oxide, which acts as a barrier against corrosion. This protective layer is crucial in preventing rust and is why zinc. How does zinc prevent corrosion? Protecting your metal surfaces with cold galvanizing; Like all metals, zinc corrodes when exposed to air and moisture. Instead, the rust continually flakes off to expose a fresh metal surface vulnerable to reaction with oxygen and water. Iron, for example, reacts with water. Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. Galvanising is a method of rust prevention.
from app.pandai.org
Iron, for example, reacts with water. However, this element does not rust like most other metals. When exposed to oxygen and moisture, zinc reacts to create zinc oxide, which acts as a barrier against corrosion. Instead, the rust continually flakes off to expose a fresh metal surface vulnerable to reaction with oxygen and water. How does zinc prevent corrosion? Like all metals, zinc corrodes when exposed to air and moisture. Galvanising is a method of rust prevention. Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. The iron or steel object is coated in a thin layer of zinc. Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products.
Rusting
How Does Zinc Stop Rust Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products. Like all metals, zinc corrodes when exposed to air and moisture. This protective layer is crucial in preventing rust and is why zinc. Galvanising is a method of rust prevention. Protecting your metal surfaces with cold galvanizing; How does zinc prevent corrosion? Instead, the rust continually flakes off to expose a fresh metal surface vulnerable to reaction with oxygen and water. However, this element does not rust like most other metals. Iron, for example, reacts with water. Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. The iron or steel object is coated in a thin layer of zinc. When exposed to oxygen and moisture, zinc reacts to create zinc oxide, which acts as a barrier against corrosion. Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products.
From www.alamy.com
The corrosion of rusted galvanized zinc is the background Stock Photo How Does Zinc Stop Rust This protective layer is crucial in preventing rust and is why zinc. Like all metals, zinc corrodes when exposed to air and moisture. Protecting your metal surfaces with cold galvanizing; Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. Instead,. How Does Zinc Stop Rust.
From dxoehamyo.blob.core.windows.net
Zinc Coating Prevents Rust at Marty Gaskin blog How Does Zinc Stop Rust When exposed to oxygen and moisture, zinc reacts to create zinc oxide, which acts as a barrier against corrosion. Instead, the rust continually flakes off to expose a fresh metal surface vulnerable to reaction with oxygen and water. Iron, for example, reacts with water. Like all metals, zinc corrodes when exposed to air and moisture. This protective layer is crucial. How Does Zinc Stop Rust.
From peacecommission.kdsg.gov.ng
What Is Zinc Cold Galvanizig Spray? Does Zinc Spray Stop Rust? One How Does Zinc Stop Rust Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. Like all metals, zinc corrodes when exposed to air and moisture. Galvanising is a method of rust prevention. How does zinc prevent corrosion? This protective layer is crucial in preventing rust. How Does Zinc Stop Rust.
From blog.thepipingmart.com
Can 304 Stainless Steel Rust? How Does Zinc Stop Rust Like all metals, zinc corrodes when exposed to air and moisture. Instead, the rust continually flakes off to expose a fresh metal surface vulnerable to reaction with oxygen and water. Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products. Iron, for example, reacts with water. The iron or steel object is coated in. How Does Zinc Stop Rust.
From studylib.net
How to Prevent White Rust on Zinc Coated Steel How Does Zinc Stop Rust Like all metals, zinc corrodes when exposed to air and moisture. The iron or steel object is coated in a thin layer of zinc. Instead, the rust continually flakes off to expose a fresh metal surface vulnerable to reaction with oxygen and water. However, this element does not rust like most other metals. Galvanising is a method of rust prevention.. How Does Zinc Stop Rust.
From grossgrow.weebly.com
Does zinc rust grossgrow How Does Zinc Stop Rust Protecting your metal surfaces with cold galvanizing; Galvanising is a method of rust prevention. Like all metals, zinc corrodes when exposed to air and moisture. How does zinc prevent corrosion? Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products. This protective layer is crucial in preventing rust and is why zinc. Iron, for. How Does Zinc Stop Rust.
From arenatews.weebly.com
Does zinc rust arenatews How Does Zinc Stop Rust Galvanising is a method of rust prevention. How does zinc prevent corrosion? Protecting your metal surfaces with cold galvanizing; When exposed to oxygen and moisture, zinc reacts to create zinc oxide, which acts as a barrier against corrosion. Like all metals, zinc corrodes when exposed to air and moisture. Overall, zinc plating provides essential protection against rust and extends the. How Does Zinc Stop Rust.
From exoznpzda.blob.core.windows.net
How Does Grease Prevent Rust at Eduardo Desantis blog How Does Zinc Stop Rust When exposed to oxygen and moisture, zinc reacts to create zinc oxide, which acts as a barrier against corrosion. This protective layer is crucial in preventing rust and is why zinc. However, this element does not rust like most other metals. Galvanising is a method of rust prevention. Zinc rusting is mostly referred to as the corrosion iron and its. How Does Zinc Stop Rust.
From decoprod.com
Does Zinc Die Cast Rust? Deco Products Zinc Die Casting How Does Zinc Stop Rust When exposed to oxygen and moisture, zinc reacts to create zinc oxide, which acts as a barrier against corrosion. Instead, the rust continually flakes off to expose a fresh metal surface vulnerable to reaction with oxygen and water. This protective layer is crucial in preventing rust and is why zinc. Like all metals, zinc corrodes when exposed to air and. How Does Zinc Stop Rust.
From sendcutsend.com
Zinc Plating vs Powder Coating for Corrosion Resistance SendCutSend How Does Zinc Stop Rust Protecting your metal surfaces with cold galvanizing; Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products. Galvanising is a method of rust prevention. Like all metals, zinc corrodes when exposed to air and moisture. How does zinc prevent corrosion? This protective layer is crucial in preventing rust and is why zinc. Iron, for. How Does Zinc Stop Rust.
From www.slideserve.com
PPT Extraction of metals PowerPoint Presentation ID208800 How Does Zinc Stop Rust Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products. The iron or steel object is coated in a thin layer of zinc. Iron, for example, reacts with water. This protective layer is crucial in preventing rust and is why zinc. Instead, the rust continually flakes off to expose a fresh metal surface vulnerable. How Does Zinc Stop Rust.
From tuvalminimal.com
How to protect SteelWindows from rust What’s the difference between How Does Zinc Stop Rust Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products. Iron, for example, reacts with water. Protecting your metal surfaces with cold galvanizing; However, this element does not rust like most other metals. The iron or steel object is coated in a thin layer of zinc. Instead, the rust continually flakes off to expose. How Does Zinc Stop Rust.
From arenatews.weebly.com
Does zinc rust arenatews How Does Zinc Stop Rust Instead, the rust continually flakes off to expose a fresh metal surface vulnerable to reaction with oxygen and water. Protecting your metal surfaces with cold galvanizing; The iron or steel object is coated in a thin layer of zinc. How does zinc prevent corrosion? This protective layer is crucial in preventing rust and is why zinc. Like all metals, zinc. How Does Zinc Stop Rust.
From dxoehamyo.blob.core.windows.net
Zinc Coating Prevents Rust at Marty Gaskin blog How Does Zinc Stop Rust This protective layer is crucial in preventing rust and is why zinc. Galvanising is a method of rust prevention. Protecting your metal surfaces with cold galvanizing; How does zinc prevent corrosion? Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process.. How Does Zinc Stop Rust.
From skwroof.com
How Does AluminumZinc coating Prevent Rust? And Its Application in How Does Zinc Stop Rust Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. Like all metals, zinc corrodes when exposed to air and moisture. When exposed to oxygen and moisture, zinc reacts to create zinc oxide, which acts as a barrier against corrosion. However,. How Does Zinc Stop Rust.
From www.youtube.com
Remove Zinc Coating From Metal The Safe and Easy Way YouTube How Does Zinc Stop Rust Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. The iron or steel object is coated in a thin layer of zinc. Iron, for example, reacts with water. Protecting your metal surfaces with cold galvanizing; When exposed to oxygen and. How Does Zinc Stop Rust.
From www.youtube.com
Rust to Zinc Electroplating Small Car Parts DIY Beginner's Full Guide How Does Zinc Stop Rust Instead, the rust continually flakes off to expose a fresh metal surface vulnerable to reaction with oxygen and water. Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. However, this element does not rust like most other metals. The iron. How Does Zinc Stop Rust.
From neotechcoatings.com
The Difference Between Rust Grip and Zinc Corrosion Coatings How Does Zinc Stop Rust Galvanising is a method of rust prevention. How does zinc prevent corrosion? Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. However, this element does not rust like most other metals. When exposed to oxygen and moisture, zinc reacts to. How Does Zinc Stop Rust.
From medium.com
4S Zinc Galvanizing Spray is a highly effective Zincrich touchup How Does Zinc Stop Rust This protective layer is crucial in preventing rust and is why zinc. Iron, for example, reacts with water. Like all metals, zinc corrodes when exposed to air and moisture. Protecting your metal surfaces with cold galvanizing; Instead, the rust continually flakes off to expose a fresh metal surface vulnerable to reaction with oxygen and water. Overall, zinc plating provides essential. How Does Zinc Stop Rust.
From www.fastenersart.com
White Rust on Zinc Plated Parts Causes, Prevention, and Solutions How Does Zinc Stop Rust This protective layer is crucial in preventing rust and is why zinc. Like all metals, zinc corrodes when exposed to air and moisture. Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products. How does zinc prevent corrosion? Iron, for example, reacts with water. Galvanising is a method of rust prevention. Instead, the rust. How Does Zinc Stop Rust.
From www.youtube.com
Nissan 350Z Rust Prevention Pt.3 Zinc Plating Guide YouTube How Does Zinc Stop Rust Like all metals, zinc corrodes when exposed to air and moisture. When exposed to oxygen and moisture, zinc reacts to create zinc oxide, which acts as a barrier against corrosion. The iron or steel object is coated in a thin layer of zinc. How does zinc prevent corrosion? Protecting your metal surfaces with cold galvanizing; Zinc rusting is mostly referred. How Does Zinc Stop Rust.
From www.youtube.com
Fast, Easy Removal of Zinc Plating. What does Wet Blasting do to Zinc How Does Zinc Stop Rust Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products. Iron, for example, reacts with water. Protecting your metal surfaces with cold galvanizing; How does zinc prevent corrosion? However, this element does not rust like most other metals. The iron or steel object is coated in a thin layer of zinc. Galvanising is a. How Does Zinc Stop Rust.
From klaxgxdtw.blob.core.windows.net
How To Stop Galvanised Steel From Rusting at William Albers blog How Does Zinc Stop Rust Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. The iron or steel object is coated in a thin layer of zinc. Like all metals, zinc corrodes when exposed to air and moisture. When exposed to oxygen and moisture, zinc. How Does Zinc Stop Rust.
From app.pandai.org
Rusting How Does Zinc Stop Rust Protecting your metal surfaces with cold galvanizing; When exposed to oxygen and moisture, zinc reacts to create zinc oxide, which acts as a barrier against corrosion. Iron, for example, reacts with water. Like all metals, zinc corrodes when exposed to air and moisture. The iron or steel object is coated in a thin layer of zinc. How does zinc prevent. How Does Zinc Stop Rust.
From blog.thepipingmart.com
Does Zinc Rust? How Does Zinc Stop Rust Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. Instead, the rust continually flakes off to expose a fresh metal surface vulnerable to reaction with oxygen and water. Overall, zinc plating provides essential protection against rust and extends the lifespan. How Does Zinc Stop Rust.
From peacecommission.kdsg.gov.ng
What Is Zinc Cold Galvanizig Spray? Does Zinc Spray Stop Rust? One How Does Zinc Stop Rust The iron or steel object is coated in a thin layer of zinc. Like all metals, zinc corrodes when exposed to air and moisture. How does zinc prevent corrosion? When exposed to oxygen and moisture, zinc reacts to create zinc oxide, which acts as a barrier against corrosion. Galvanising is a method of rust prevention. Overall, zinc plating provides essential. How Does Zinc Stop Rust.
From dxoehamyo.blob.core.windows.net
Zinc Coating Prevents Rust at Marty Gaskin blog How Does Zinc Stop Rust Like all metals, zinc corrodes when exposed to air and moisture. This protective layer is crucial in preventing rust and is why zinc. Protecting your metal surfaces with cold galvanizing; Galvanising is a method of rust prevention. How does zinc prevent corrosion? Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products. Instead, the. How Does Zinc Stop Rust.
From housinghow.com
Does Zinc Roofing Rust? (Read This First) [2024] How Does Zinc Stop Rust Like all metals, zinc corrodes when exposed to air and moisture. When exposed to oxygen and moisture, zinc reacts to create zinc oxide, which acts as a barrier against corrosion. The iron or steel object is coated in a thin layer of zinc. Galvanising is a method of rust prevention. Overall, zinc plating provides essential protection against rust and extends. How Does Zinc Stop Rust.
From peacecommission.kdsg.gov.ng
What Is Zinc Cold Galvanizig Spray? Does Zinc Spray Stop Rust? One How Does Zinc Stop Rust The iron or steel object is coated in a thin layer of zinc. Iron, for example, reacts with water. However, this element does not rust like most other metals. How does zinc prevent corrosion? Like all metals, zinc corrodes when exposed to air and moisture. Galvanising is a method of rust prevention. Protecting your metal surfaces with cold galvanizing; This. How Does Zinc Stop Rust.
From www.youtube.com
Air compressor tank rust proofing YouTube How Does Zinc Stop Rust How does zinc prevent corrosion? This protective layer is crucial in preventing rust and is why zinc. Iron, for example, reacts with water. Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. Like all metals, zinc corrodes when exposed to. How Does Zinc Stop Rust.
From www.superior-tech.net
How zinc coating and the galvanizing process protects iron from rust How Does Zinc Stop Rust Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. However, this element does not rust like most other metals. Galvanising is a method of rust prevention. Like all metals, zinc corrodes when exposed to air and moisture. Protecting your metal. How Does Zinc Stop Rust.
From sciencenotes.org
What Is Galvanization? Does Galvanized Steel Rust? How Does Zinc Stop Rust Iron, for example, reacts with water. How does zinc prevent corrosion? However, this element does not rust like most other metals. Galvanising is a method of rust prevention. The iron or steel object is coated in a thin layer of zinc. Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products. Instead, the rust. How Does Zinc Stop Rust.
From rotechrural.com.au
Zinc Plated vs Galvanised What’s The Difference? Rotech Rural How Does Zinc Stop Rust Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. Protecting your metal surfaces with cold galvanizing; How does zinc prevent corrosion? Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products. When exposed to. How Does Zinc Stop Rust.
From www.dreamstime.com
Zinc rust stock image. Image of dirty, pattern, wall 112541715 How Does Zinc Stop Rust Overall, zinc plating provides essential protection against rust and extends the lifespan of many metal products. Like all metals, zinc corrodes when exposed to air and moisture. Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc oxidation refers to the process. The iron or steel. How Does Zinc Stop Rust.
From exohbxkrs.blob.core.windows.net
How To Prevent Rust On Galvanized Steel at Chase Polen blog How Does Zinc Stop Rust Like all metals, zinc corrodes when exposed to air and moisture. When exposed to oxygen and moisture, zinc reacts to create zinc oxide, which acts as a barrier against corrosion. Iron, for example, reacts with water. Zinc rusting is mostly referred to as the corrosion iron and its alloys when it reacts with oxygen, and water among others while zinc. How Does Zinc Stop Rust.