Standard Potential Of Lithium . 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. Standard potential e ° (volts): Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential. The more positive the potential, the more likely the. It is capable of reducing any substance above on the table. When lithium acts a reducing agent, you reverse the equation and the charge listed in the table, giving the reaction a potential of +3.05 volts. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode.
from cpb.iphy.ac.cn
It is capable of reducing any substance above on the table. The more positive the potential, the more likely the. 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. Standard potential e ° (volts): As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. When lithium acts a reducing agent, you reverse the equation and the charge listed in the table, giving the reaction a potential of +3.05 volts.
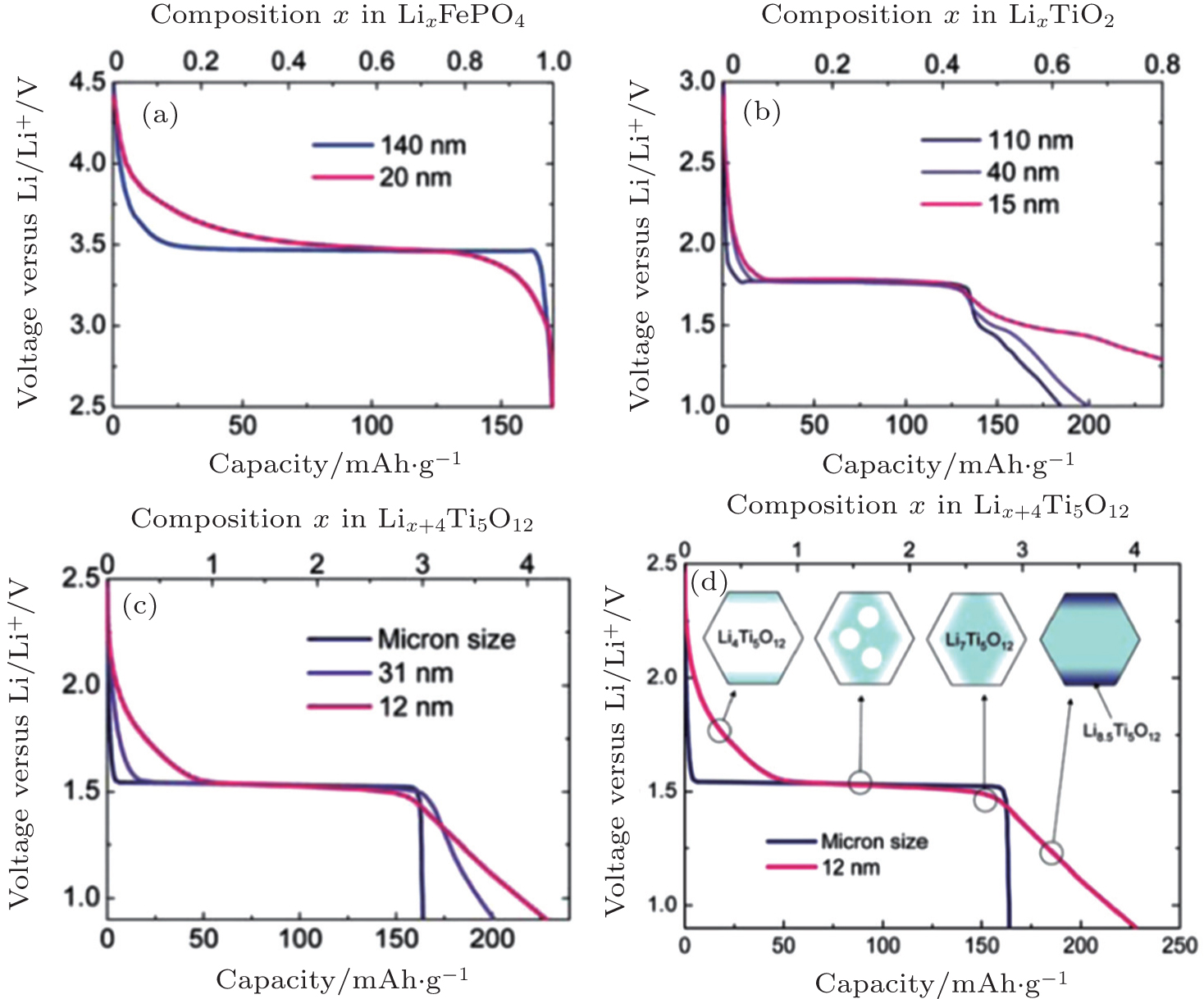
Brief overview of electrochemical potential in lithium ion batteries
Standard Potential Of Lithium As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. It is capable of reducing any substance above on the table. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Standard potential e ° (volts): 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. The more positive the potential, the more likely the. As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. When lithium acts a reducing agent, you reverse the equation and the charge listed in the table, giving the reaction a potential of +3.05 volts. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential.
From pubs.acs.org
Ab Initio Calculations of the Redox Potentials of Additives for Lithium Standard Potential Of Lithium Standard potential e ° (volts): The more positive the potential, the more likely the. When lithium acts a reducing agent, you reverse the equation and the charge listed in the table, giving the reaction a potential of +3.05 volts. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. The. Standard Potential Of Lithium.
From www.mdpi.com
Processes Free FullText A Review of LithiumIon Battery Thermal Standard Potential Of Lithium It is capable of reducing any substance above on the table. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. Standard potential e ° (volts): As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. Lithium metal is the. Standard Potential Of Lithium.
From cpb.iphy.ac.cn
Brief overview of electrochemical potential in lithium ion batteries Standard Potential Of Lithium The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. It is capable of reducing any substance above on the table. When lithium acts a reducing agent, you reverse the equation and the charge listed in the table, giving the reaction a potential of +3.05 volts. 372. Standard Potential Of Lithium.
From www.researchgate.net
Potentials of metals in various solutions (vs SHE) Download Table Standard Potential Of Lithium As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. When lithium acts a reducing agent, you reverse. Standard Potential Of Lithium.
From www.researchgate.net
Comparison between gravimetric and volumetric capacities, standard Standard Potential Of Lithium The more positive the potential, the more likely the. Standard potential e ° (volts): The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential. It. Standard Potential Of Lithium.
From www.researchgate.net
(A) Comparison of the abundance of Li, Na, and K resources with the Standard Potential Of Lithium It is capable of reducing any substance above on the table. As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. When lithium acts a reducing agent, you reverse the equation and the charge listed in the table,. Standard Potential Of Lithium.
From www.semanticscholar.org
Silicon as a potential anode material for Liion batteries where size Standard Potential Of Lithium The more positive the potential, the more likely the. Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential. It is capable of reducing any substance above on the table. 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. As an element with one of. Standard Potential Of Lithium.
From www.researchgate.net
CV and redox potential for the Fe(III)/Fe(II) redox couple of different Standard Potential Of Lithium Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential. As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. It is capable of reducing any substance above on the table. Standard potential e ° (volts): The standard. Standard Potential Of Lithium.
From www.researchgate.net
Equilibrium potential vs. Li (V) as a function of stoichiometric Standard Potential Of Lithium The more positive the potential, the more likely the. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest. Standard Potential Of Lithium.
From www.chegg.com
Solved Calculate the standard cell potential of the Standard Potential Of Lithium As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential. When lithium acts a reducing agent, you reverse the equation and the charge listed in the table, giving. Standard Potential Of Lithium.
From byjus.com
Is oxidation potential always positive and reduction potential always Standard Potential Of Lithium 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Standard potential e ° (volts): The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. The more. Standard Potential Of Lithium.
From www.chegg.com
Solved Refer to the table of standard reduction potentials Standard Potential Of Lithium The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. Standard potential e ° (volts): Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential. 372. Standard Potential Of Lithium.
From medium.com
Lithium plating. This summary page is largely based on… by Roman Standard Potential Of Lithium 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. The more positive the potential, the more likely the. When lithium acts a reducing agent, you reverse the equation and the charge listed in the table, giving the reaction a potential of +3.05 volts. Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah. Standard Potential Of Lithium.
From cpb.iphy.ac.cn
Brief overview of electrochemical potential in lithium ion batteries Standard Potential Of Lithium The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential. As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential. Standard Potential Of Lithium.
From cpb.iphy.ac.cn
Brief overview of electrochemical potential in lithium ion batteries Standard Potential Of Lithium The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. The more positive the potential, the more likely the. It is capable of reducing any substance above on the table. When lithium acts a reducing agent, you reverse. Standard Potential Of Lithium.
From www.researchgate.net
In Lithium ion batteries with graphite anode, what would be the final Standard Potential Of Lithium The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. It is capable of reducing any substance above on the table. Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential. The standard reduction potential. Standard Potential Of Lithium.
From www.mdpi.com
Processes Free FullText Recent Advances in Lithium Extraction Standard Potential Of Lithium The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. It is capable of reducing any substance above on the table. As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. When lithium acts a reducing agent, you reverse the. Standard Potential Of Lithium.
From abdoelazizz767.blogspot.com
2 Chemicals In Lithium Ion Batteries J Compos Sci Free Full Text A Standard Potential Of Lithium Standard potential e ° (volts): The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The table is ordered such that the stronger (more reactive) reductants are at the. Standard Potential Of Lithium.
From cpb.iphy.ac.cn
Brief overview of electrochemical potential in lithium ion batteries Standard Potential Of Lithium The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. When lithium acts a reducing. Standard Potential Of Lithium.
From www.flinnsci.com
Standard Reduction Potential Charts for Chemistry Standard Potential Of Lithium 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. It is capable of reducing any substance above on the table. As an element with one. Standard Potential Of Lithium.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu Standard Potential Of Lithium The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. The more positive the potential,. Standard Potential Of Lithium.
From www.toppr.com
The standard reduction potential 25^oC of Li^+/Li,,Ba^{2+}/Ba,,Na Standard Potential Of Lithium Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen. Standard Potential Of Lithium.
From www.allaboutcircuits.com
Quantum Physics Solidstate Device Theory Electronics Textbook Standard Potential Of Lithium Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential. When lithium acts a reducing agent, you reverse the equation and the charge listed in the table, giving the reaction a potential of +3.05 volts. 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. As. Standard Potential Of Lithium.
From www.researchgate.net
a) Abundance of Li, Na, and K metal in Earth's crust (wt. ); b Standard Potential Of Lithium 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. The more positive the potential, the more likely the. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an. Standard Potential Of Lithium.
From www.chegg.com
Solved List the electrochemical series you developed from Standard Potential Of Lithium The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential. It is capable of reducing any substance above on the table. As an element with. Standard Potential Of Lithium.
From mccord.cm.utexas.edu
Standard Potential Standard Potential Of Lithium When lithium acts a reducing agent, you reverse the equation and the charge listed in the table, giving the reaction a potential of +3.05 volts. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Standard potential e ° (volts): 42 rows lithium metal \(\left( \ce{li} \right)\). Standard Potential Of Lithium.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Potential Of Lithium As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential. Standard potential e ° (volts): The standard reduction potential can be determined by subtracting the standard reduction potential. Standard Potential Of Lithium.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Standard Potential Of Lithium Standard potential e ° (volts): As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. When lithium acts a reducing agent, you reverse the equation and the. Standard Potential Of Lithium.
From www.toppr.com
The standard reduction potentials at 25^∘C of Li^+/Li, Ba^2 + /Ba, Na Standard Potential Of Lithium The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. It is capable of reducing any substance above on the table. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. When lithium acts a reducing. Standard Potential Of Lithium.
From techxplore.com
Researchers design nextgeneration electrolytes for lithium metal batteries Standard Potential Of Lithium 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Standard potential e ° (volts): The more positive the potential, the more likely the. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. When lithium. Standard Potential Of Lithium.
From www.pinterest.co.kr
pe=log[e] measuved in volts.if pe is negative reduced, if pe is Standard Potential Of Lithium Lithium metal is the lightest metal and possesses a high specific capacity (3.86 ah g −1) and an extremely low electrode potential. When lithium acts a reducing agent, you reverse the equation and the charge listed in the table, giving the reaction a potential of +3.05 volts. It is capable of reducing any substance above on the table. As an. Standard Potential Of Lithium.
From inspiritvr.com
Calculating Standard Cell Potentials Study Guide Inspirit Standard Potential Of Lithium 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. Standard potential e ° (volts): When lithium acts a reducing agent, you reverse the equation and the charge listed in the table, giving the reaction a potential of +3.05 volts. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger. Standard Potential Of Lithium.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID4831905 Standard Potential Of Lithium As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 42 rows lithium metal \(\left( \ce{li} \right)\) is the strongest reducing agent. Standard potential e ° (volts): Lithium metal. Standard Potential Of Lithium.
From www.infenety.com
NextGeneration Electrolytes for High Energy Density Lithium Metal Standard Potential Of Lithium The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode. It is capable of reducing any substance above on the table. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Lithium metal is the lightest metal and possesses. Standard Potential Of Lithium.
From www.chegg.com
Solved 5. (a) Use the standard reduction potentials at 25° C Standard Potential Of Lithium 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. As an element with one of the lowest electronegativity (χ = 0.98) and most negative standard electrode potential (e =. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the. Standard Potential Of Lithium.