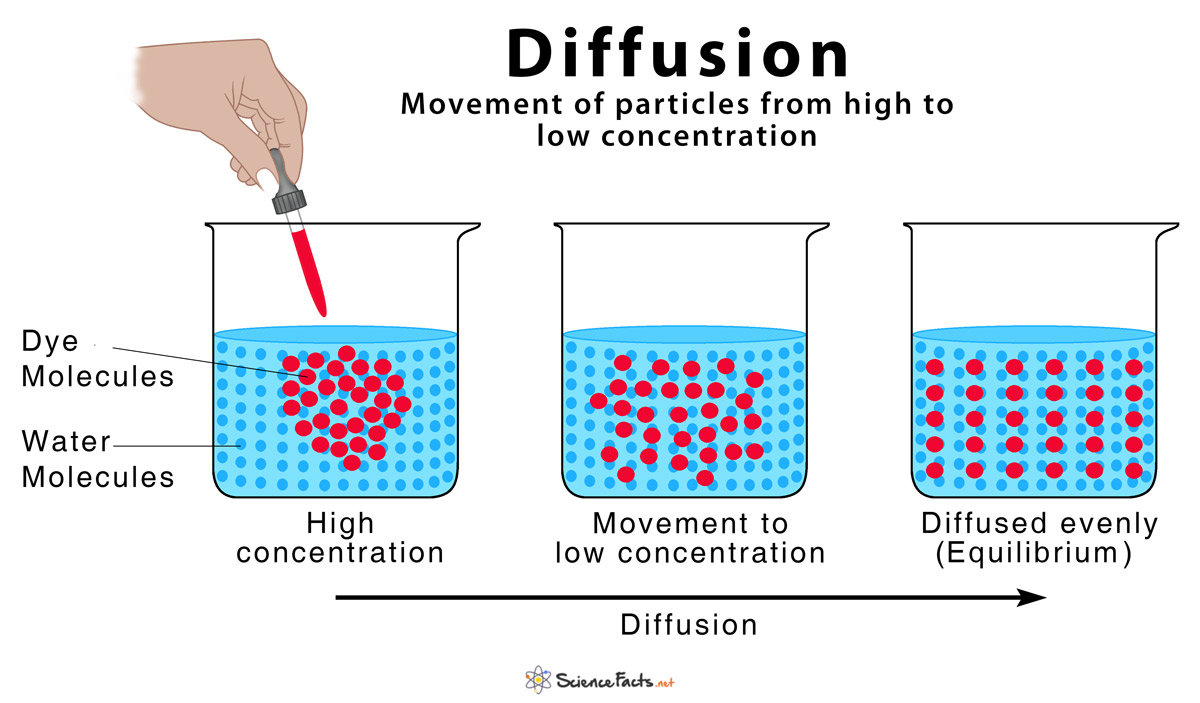
Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples . Warmer the temperature, higher is the rate of diffusion. Diffusion occurs in gases and liquids, due to the random motion of their particles. Diffusion happens on its own when the particles spread out from an. Spreading out and mixing of one substance with other is called diffusion. It is where particles move from an area of high. Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. The density of a gas is equal to the mass of the gas divided by the volume of the gas. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. If the volume is held constant one gas is compared. The different factors that affect diffusion either individually or collectively are:
from www.sciencefacts.net
Spreading out and mixing of one substance with other is called diffusion. Diffusion occurs in gases and liquids, due to the random motion of their particles. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. The different factors that affect diffusion either individually or collectively are: Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. The density of a gas is equal to the mass of the gas divided by the volume of the gas. If the volume is held constant one gas is compared. Warmer the temperature, higher is the rate of diffusion. It is where particles move from an area of high. Diffusion happens on its own when the particles spread out from an.
Diffusion Definition and How Does it Occur (with Diagram)
Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. It is where particles move from an area of high. The density of a gas is equal to the mass of the gas divided by the volume of the gas. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Diffusion occurs in gases and liquids, due to the random motion of their particles. Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. Diffusion happens on its own when the particles spread out from an. Spreading out and mixing of one substance with other is called diffusion. The different factors that affect diffusion either individually or collectively are: If the volume is held constant one gas is compared. Warmer the temperature, higher is the rate of diffusion.
From talanewtanthony.blogspot.com
Solids Liquids and Gases TalanewtAnthony Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Diffusion happens on its own when the particles spread out from an. Warmer the temperature, higher is the rate of diffusion. The density of a gas is equal to the mass of the gas divided by the volume of the gas. Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a.. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples If the volume is held constant one gas is compared. Spreading out and mixing of one substance with other is called diffusion. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Diffusion occurs in gases and liquids, due to the random motion of their particles. Diffusion happens on its own. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.youtube.com
Diffusion in Solids, liquids and Gases ClassIX in Hindi easy Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples The density of a gas is equal to the mass of the gas divided by the volume of the gas. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. Diffusion happens. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From courses.lumenlearning.com
8.2 Solids and Liquids The Basics of General, Organic, and Biological Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. Diffusion occurs in gases and liquids, due to the random motion of their particles. Diffusion happens on its own when the particles spread out from an. If the volume is held constant one gas is compared. The density of a gas. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From sciencetallis.weebly.com
3. Particle Model of Matter THOMAS TALLIS SCIENCE Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. If the volume is held constant one gas is compared. Diffusion occurs in gases and liquids, due to the random motion of their particles. The density of a gas is equal to the mass of the gas divided by the volume. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From psiberg.com
Properties of Solid, Liquid, Gases A Comparison Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples If the volume is held constant one gas is compared. Diffusion occurs in gases and liquids, due to the random motion of their particles. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. It is where particles move from an area of high. Fick’s laws of diffusion are mathematical statements. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From slideplayer.com
Diffusion D. Crowley, ppt download Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. Diffusion occurs in gases and liquids, due to the random motion of their particles. If the volume is held constant one gas is compared. The density of a gas is equal to the mass of the gas divided by the volume. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.expii.com
Diffusion and Effusion — Definition & Overview Expii Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Diffusion occurs in gases and liquids, due to the random motion of their particles. It is where particles move from an area of high. Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. The different factors that affect diffusion either individually or collectively are: Warmer the temperature, higher is the. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. If the volume is held constant one gas is compared. Spreading out and mixing of one substance with other is called diffusion.. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.slideserve.com
PPT Solids, liquids and gases PowerPoint Presentation, free download Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Spreading out and mixing of one substance with other is called diffusion. If the volume is held constant one gas is compared. The different factors that affect diffusion either individually or collectively are: The density of a gas is equal to the mass of the gas divided by the volume of the gas. Diffusion is how smells spread out through. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From mungfali.com
Solids Liquids Gases Chart Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Diffusion happens on its own when the particles spread out from an. If the volume is held constant one gas is compared. Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. Diffusion occurs in gases and liquids, due to the random motion of their particles. Warmer the temperature, higher is. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.majordifferences.com
Difference between Solid, Liquid and Gas Table (Solids vs Liquids vs Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. Warmer the temperature, higher is the rate of diffusion. Spreading out and mixing of one substance with other is called diffusion. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. The. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.teachoo.com
Diffusion in Solids, Liquids and Gases with Examples Teachoo Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Warmer the temperature, higher is the rate of diffusion. The different factors that affect diffusion either individually or collectively are: Spreading out and mixing of one substance with other is called diffusion. It is where particles move from an area of high. Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From exoetvrqy.blob.core.windows.net
Solids Liquids And Gases Examples at Edward Yuan blog Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Diffusion happens on its own when the particles spread out from an. Spreading out and mixing of one substance with other is called diffusion. The density of a gas is equal to the mass of the gas divided by the volume of the gas. Diffusion is how smells spread out through the air and how concentrated liquids spread out when. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From oneseds.blogspot.com
Gas Particle Diagram Onesed Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples The different factors that affect diffusion either individually or collectively are: It is where particles move from an area of high. Spreading out and mixing of one substance with other is called diffusion. If the volume is held constant one gas is compared. Diffusion happens on its own when the particles spread out from an. Fick’s laws of diffusion are. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.slideserve.com
PPT CHAPTER 5 DIFFUSION IN SOLIDS PowerPoint Presentation, free Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples It is where particles move from an area of high. Diffusion occurs in gases and liquids, due to the random motion of their particles. If the volume is held constant one gas is compared. The density of a gas is equal to the mass of the gas divided by the volume of the gas. The different factors that affect diffusion. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Warmer the temperature, higher is the rate of diffusion. Spreading out and mixing of one substance with other is called diffusion. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. The different factors that affect diffusion either individually or collectively are: It is where particles move from an area of. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From dokumen.tips
(PDF) States of Matter · being Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples The different factors that affect diffusion either individually or collectively are: Warmer the temperature, higher is the rate of diffusion. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Diffusion happens on its own when the particles spread out from an. Fick’s laws of diffusion are mathematical statements describing how. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From brainly.in
Illustrate an experiment how diffusion in liquid can be demonstrated Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Diffusion happens on its own when the particles spread out from an. The different factors that affect diffusion either individually or collectively are: The density of a gas is equal to the mass of the gas divided by the volume of the gas. Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.shutterstock.com
Diffusion solids liquids Images, Stock Photos & Vectors Shutterstock Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. It is where particles move from an area of high. The different factors that affect diffusion either individually or collectively are: Diffusion occurs in gases and liquids, due to the random motion of their particles. The density of a gas is. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.teachoo.com
Diffusion in Solids, Liquids and Gases with Examples Teachoo Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Diffusion occurs in gases and liquids, due to the random motion of their particles. Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. Spreading out and mixing of one substance with. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples If the volume is held constant one gas is compared. The different factors that affect diffusion either individually or collectively are: Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. Diffusion occurs in gases and liquids, due to the random motion of their particles. Warmer the temperature, higher is the. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.thoughtco.com
Examples of Diffusion in Chemistry Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Diffusion occurs in gases and liquids, due to the random motion of their particles. It is where particles move from an area of high. The density of a gas is equal to the mass of the gas divided by the volume of the gas. Warmer the temperature, higher is the rate of diffusion. Diffusion is how smells spread out through. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From learningspeedos.z13.web.core.windows.net
Picture Of Solid Liquid And Gas Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples The density of a gas is equal to the mass of the gas divided by the volume of the gas. Warmer the temperature, higher is the rate of diffusion. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. If the volume is held constant one gas is compared. It is. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From byjus.com
What is diffusion? Explain about diffusion in solid , liquid and gas Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples The density of a gas is equal to the mass of the gas divided by the volume of the gas. If the volume is held constant one gas is compared. Warmer the temperature, higher is the rate of diffusion. Diffusion happens on its own when the particles spread out from an. The different factors that affect diffusion either individually or. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.slideserve.com
PPT Water and its relation to Diffusion and Osmosis PowerPoint Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples The different factors that affect diffusion either individually or collectively are: The density of a gas is equal to the mass of the gas divided by the volume of the gas. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Fick’s laws of diffusion are mathematical statements describing how particles. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.teachoo.com
Diffusion in Solids, Liquids and Gases with Examples Teachoo Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Diffusion occurs in gases and liquids, due to the random motion of their particles. Warmer the temperature, higher is the rate of diffusion. Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From mungfali.com
Solids Liquids Gases Chart Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Spreading out and mixing of one substance with other is called diffusion. If the volume is held constant one gas is compared. It is where particles move from an area of high. The density of a gas is equal to the mass of the gas divided by the volume of the gas. Diffusion is how smells spread out through the. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.jove.com
11340.jpg Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. If the volume is held constant one gas is compared. Diffusion occurs in gases and liquids, due to the random motion of their particles. Diffusion happens on its own when the particles spread out from an. The different factors that affect. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From diagramlisthavens.z21.web.core.windows.net
Solid Liquid And Gas Diagram Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Diffusion happens on its own when the particles spread out from an. It is where particles move from an area of high. Warmer the temperature, higher is the rate of diffusion. The density of a gas is equal to the mass. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From diffuserjaika.blogspot.com
5 Examples Of Diffusion Gas Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Diffusion happens on its own when the particles spread out from an. The density of a gas is equal to the mass of the gas divided by the volume of the gas. Diffusion occurs in gases and liquids, due to the random motion of their particles. It is where particles move from an area of high. If the volume is. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.youtube.com
Diffusion in Solid Chemistry YouTube Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Spreading out and mixing of one substance with other is called diffusion. It is where particles move from an area of high. Warmer the temperature, higher is the rate of diffusion. The density of a gas is equal to the mass of the gas divided by the volume of the gas. Diffusion is how smells spread out through the air. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From byjus.com
Give a simple activity/experiment to show that the rate of diffusion Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Fick’s laws of diffusion are mathematical statements describing how particles under random thermal motion tend to spread from a. The different factors that affect diffusion either individually or collectively are: The density of a gas is equal to the mass of. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From www.sciencefacts.net
Diffusion Definition and How Does it Occur (with Diagram) Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Warmer the temperature, higher is the rate of diffusion. Diffusion happens on its own when the particles spread out from an. Diffusion occurs in gases and liquids, due to the random motion of their particles. The different factors that affect diffusion. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples The density of a gas is equal to the mass of the gas divided by the volume of the gas. Diffusion happens on its own when the particles spread out from an. Diffusion occurs in gases and liquids, due to the random motion of their particles. The different factors that affect diffusion either individually or collectively are: Spreading out and. Explain The Rate Of Diffusion In Solids Liquids And Gases With Examples.