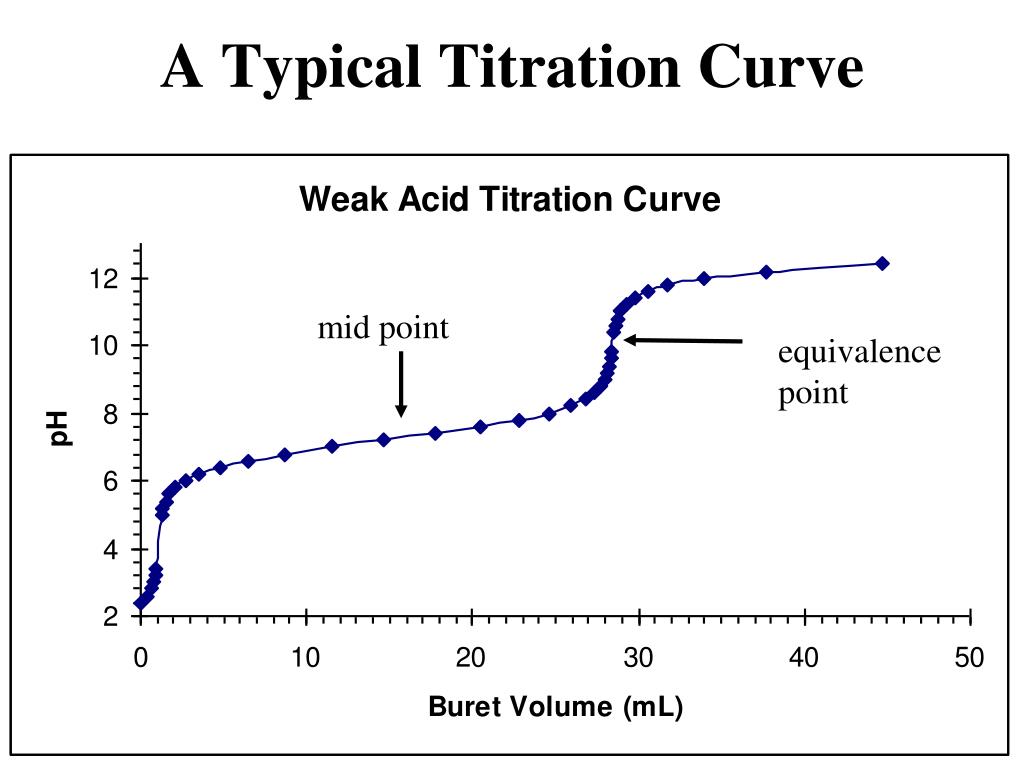
Titration Curve Questions And Answers Pdf . If the acids/bases are strong or weak strong. Practicals for papers 3 and 5. (1) a fairly flat “buffered” region at low ph; Such a curve has three re‐ gions: From the graph at the right, determine a. Consider the titration of a weak acid, ha, with a strong base that gives the following ph curve. We haven’t added any naoh, so the ph. Titration curve practice problems (key) 1. This question is about acids and bases. Volume of titrant (solution in the buret). A titration curve is a plot of solution ph in a flask vs. Titrating a weak acid (hcn) with a strong base (naoh. (2) a steep “equivalence point” region at. Figure 1 shows a titration curve. Use the information to determine the concentration of the hydrochloric acid.
from www.slideserve.com
Practicals for papers 3 and 5. (2) a steep “equivalence point” region at. Consider the titration of a weak acid, ha, with a strong base that gives the following ph curve. Use the information to determine the concentration of the hydrochloric acid. From the graph at the right, determine a. A titration curve is a plot of solution ph in a flask vs. This question is about acids and bases. Titration curve practice problems (key) 1. We haven’t added any naoh, so the ph. (1) a fairly flat “buffered” region at low ph;
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155
Titration Curve Questions And Answers Pdf We haven’t added any naoh, so the ph. Such a curve has three re‐ gions: We haven’t added any naoh, so the ph. (1) a fairly flat “buffered” region at low ph; From the graph at the right, determine a. Use the information to determine the concentration of the hydrochloric acid. This question is about acids and bases. (2) a steep “equivalence point” region at. Titration curve practice problems (key) 1. Figure 1 shows a titration curve. Practicals for papers 3 and 5. Titrating a weak acid (hcn) with a strong base (naoh. A titration curve is a plot of solution ph in a flask vs. Volume of titrant (solution in the buret). Consider the titration of a weak acid, ha, with a strong base that gives the following ph curve. If the acids/bases are strong or weak strong.
From dokumen.tips
(PDF) Titration Curve DOKUMEN.TIPS Titration Curve Questions And Answers Pdf (2) a steep “equivalence point” region at. Such a curve has three re‐ gions: Practicals for papers 3 and 5. We haven’t added any naoh, so the ph. Use the information to determine the concentration of the hydrochloric acid. Volume of titrant (solution in the buret). If the acids/bases are strong or weak strong. Consider the titration of a weak. Titration Curve Questions And Answers Pdf.
From oneclass.com
OneClass Questions 26 27 are based on the titration curve shown below. This curve represents Titration Curve Questions And Answers Pdf Such a curve has three re‐ gions: (2) a steep “equivalence point” region at. Figure 1 shows a titration curve. Use the information to determine the concentration of the hydrochloric acid. From the graph at the right, determine a. Titrating a weak acid (hcn) with a strong base (naoh. A titration curve is a plot of solution ph in a. Titration Curve Questions And Answers Pdf.
From www.chegg.com
Solved Given the following titration curve answer questions Titration Curve Questions And Answers Pdf Figure 1 shows a titration curve. A titration curve is a plot of solution ph in a flask vs. If the acids/bases are strong or weak strong. (2) a steep “equivalence point” region at. Volume of titrant (solution in the buret). Use the information to determine the concentration of the hydrochloric acid. Consider the titration of a weak acid, ha,. Titration Curve Questions And Answers Pdf.
From www.studocu.com
Titration Curve Practice Problems MATH + SCIENCE INITIATIVE Titration Curve Practice Problems Titration Curve Questions And Answers Pdf Practicals for papers 3 and 5. Consider the titration of a weak acid, ha, with a strong base that gives the following ph curve. If the acids/bases are strong or weak strong. A titration curve is a plot of solution ph in a flask vs. This question is about acids and bases. (1) a fairly flat “buffered” region at low. Titration Curve Questions And Answers Pdf.
From www.studypool.com
SOLUTION Titration curve of strong acid with weak base Studypool Titration Curve Questions And Answers Pdf (1) a fairly flat “buffered” region at low ph; Titration curve practice problems (key) 1. (2) a steep “equivalence point” region at. Consider the titration of a weak acid, ha, with a strong base that gives the following ph curve. Practicals for papers 3 and 5. From the graph at the right, determine a. We haven’t added any naoh, so. Titration Curve Questions And Answers Pdf.
From www.chegg.com
Solved The following are Weak Acid/Strong Base Titration Titration Curve Questions And Answers Pdf We haven’t added any naoh, so the ph. (1) a fairly flat “buffered” region at low ph; Such a curve has three re‐ gions: Titration curve practice problems (key) 1. Use the information to determine the concentration of the hydrochloric acid. Figure 1 shows a titration curve. Titrating a weak acid (hcn) with a strong base (naoh. If the acids/bases. Titration Curve Questions And Answers Pdf.
From www.chegg.com
Solved 24. The figure below shows the titration curves of Titration Curve Questions And Answers Pdf A titration curve is a plot of solution ph in a flask vs. Titrating a weak acid (hcn) with a strong base (naoh. Practicals for papers 3 and 5. This question is about acids and bases. Figure 1 shows a titration curve. Titration curve practice problems (key) 1. Volume of titrant (solution in the buret). We haven’t added any naoh,. Titration Curve Questions And Answers Pdf.
From www.studypool.com
SOLUTION Titration calculations and answers Studypool Titration Curve Questions And Answers Pdf (1) a fairly flat “buffered” region at low ph; Volume of titrant (solution in the buret). We haven’t added any naoh, so the ph. A titration curve is a plot of solution ph in a flask vs. This question is about acids and bases. Consider the titration of a weak acid, ha, with a strong base that gives the following. Titration Curve Questions And Answers Pdf.
From www.studypool.com
SOLUTION Titration calculations and answers Studypool Titration Curve Questions And Answers Pdf From the graph at the right, determine a. A titration curve is a plot of solution ph in a flask vs. Titrating a weak acid (hcn) with a strong base (naoh. Consider the titration of a weak acid, ha, with a strong base that gives the following ph curve. This question is about acids and bases. Figure 1 shows a. Titration Curve Questions And Answers Pdf.
From www.chegg.com
Solved Titration Curves Worksheet The following are Weak Titration Curve Questions And Answers Pdf Consider the titration of a weak acid, ha, with a strong base that gives the following ph curve. (1) a fairly flat “buffered” region at low ph; Figure 1 shows a titration curve. Titrating a weak acid (hcn) with a strong base (naoh. Practicals for papers 3 and 5. Such a curve has three re‐ gions: Use the information to. Titration Curve Questions And Answers Pdf.
From solvedlib.com
The titration curve for Histidine is shown labeled at… SolvedLib Titration Curve Questions And Answers Pdf This question is about acids and bases. Titration curve practice problems (key) 1. A titration curve is a plot of solution ph in a flask vs. We haven’t added any naoh, so the ph. If the acids/bases are strong or weak strong. (1) a fairly flat “buffered” region at low ph; Use the information to determine the concentration of the. Titration Curve Questions And Answers Pdf.
From edurev.in
Graphical Representation of Titration Curves Chemistry for JAMB PDF Download Titration Curve Questions And Answers Pdf Volume of titrant (solution in the buret). If the acids/bases are strong or weak strong. Figure 1 shows a titration curve. A titration curve is a plot of solution ph in a flask vs. Consider the titration of a weak acid, ha, with a strong base that gives the following ph curve. Such a curve has three re‐ gions: Titration. Titration Curve Questions And Answers Pdf.
From www.chegg.com
Solved Use the titration curve below to answer the following Titration Curve Questions And Answers Pdf This question is about acids and bases. A titration curve is a plot of solution ph in a flask vs. Titration curve practice problems (key) 1. Such a curve has three re‐ gions: Use the information to determine the concentration of the hydrochloric acid. Volume of titrant (solution in the buret). Titrating a weak acid (hcn) with a strong base. Titration Curve Questions And Answers Pdf.
From www.chegg.com
Solved 5. Titration curve of an unknown amino acid The Titration Curve Questions And Answers Pdf Such a curve has three re‐ gions: Use the information to determine the concentration of the hydrochloric acid. Volume of titrant (solution in the buret). Consider the titration of a weak acid, ha, with a strong base that gives the following ph curve. This question is about acids and bases. Practicals for papers 3 and 5. Titrating a weak acid. Titration Curve Questions And Answers Pdf.
From www.coursehero.com
[Solved] . D Question 23 3 pts Please match the following titration curves... Course Hero Titration Curve Questions And Answers Pdf Practicals for papers 3 and 5. Volume of titrant (solution in the buret). From the graph at the right, determine a. We haven’t added any naoh, so the ph. This question is about acids and bases. If the acids/bases are strong or weak strong. Titration curve practice problems (key) 1. (1) a fairly flat “buffered” region at low ph; Use. Titration Curve Questions And Answers Pdf.
From www.studocu.com
Titration questions and answers 1. Titration (Higher Level) 2002 Question 1 (a) COMPOUND Titration Curve Questions And Answers Pdf We haven’t added any naoh, so the ph. This question is about acids and bases. Consider the titration of a weak acid, ha, with a strong base that gives the following ph curve. Volume of titrant (solution in the buret). Such a curve has three re‐ gions: (2) a steep “equivalence point” region at. Titration curve practice problems (key) 1.. Titration Curve Questions And Answers Pdf.
From www.chegg.com
Solved The titration curve for a weak acid/strong base Titration Curve Questions And Answers Pdf Titrating a weak acid (hcn) with a strong base (naoh. Titration curve practice problems (key) 1. From the graph at the right, determine a. This question is about acids and bases. If the acids/bases are strong or weak strong. We haven’t added any naoh, so the ph. (1) a fairly flat “buffered” region at low ph; Volume of titrant (solution. Titration Curve Questions And Answers Pdf.
From www.chegg.com
Solved Use the titration curve shown below to answer Titration Curve Questions And Answers Pdf Volume of titrant (solution in the buret). Titrating a weak acid (hcn) with a strong base (naoh. Such a curve has three re‐ gions: Practicals for papers 3 and 5. Consider the titration of a weak acid, ha, with a strong base that gives the following ph curve. Use the information to determine the concentration of the hydrochloric acid. Figure. Titration Curve Questions And Answers Pdf.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Chemistry JoVe Titration Curve Questions And Answers Pdf Use the information to determine the concentration of the hydrochloric acid. This question is about acids and bases. Titrating a weak acid (hcn) with a strong base (naoh. From the graph at the right, determine a. Consider the titration of a weak acid, ha, with a strong base that gives the following ph curve. Titration curve practice problems (key) 1.. Titration Curve Questions And Answers Pdf.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Curve Questions And Answers Pdf This question is about acids and bases. Figure 1 shows a titration curve. Practicals for papers 3 and 5. A titration curve is a plot of solution ph in a flask vs. From the graph at the right, determine a. (2) a steep “equivalence point” region at. (1) a fairly flat “buffered” region at low ph; Use the information to. Titration Curve Questions And Answers Pdf.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Questions And Answers Pdf Titration curve practice problems (key) 1. A titration curve is a plot of solution ph in a flask vs. From the graph at the right, determine a. Figure 1 shows a titration curve. (1) a fairly flat “buffered” region at low ph; Titrating a weak acid (hcn) with a strong base (naoh. Such a curve has three re‐ gions: Practicals. Titration Curve Questions And Answers Pdf.
From www.studypool.com
SOLUTION Chemsheets gcse 1105 titrations 1 ans 93ghs Studypool Titration Curve Questions And Answers Pdf We haven’t added any naoh, so the ph. From the graph at the right, determine a. Volume of titrant (solution in the buret). Such a curve has three re‐ gions: If the acids/bases are strong or weak strong. Practicals for papers 3 and 5. (2) a steep “equivalence point” region at. Titration curve practice problems (key) 1. Use the information. Titration Curve Questions And Answers Pdf.
From studyfinder.org
The Ultimate Guide to Understanding and Interpreting Titration Curves POGIL Answers Revealed! Titration Curve Questions And Answers Pdf (2) a steep “equivalence point” region at. A titration curve is a plot of solution ph in a flask vs. This question is about acids and bases. Titrating a weak acid (hcn) with a strong base (naoh. Figure 1 shows a titration curve. Volume of titrant (solution in the buret). We haven’t added any naoh, so the ph. If the. Titration Curve Questions And Answers Pdf.
From www.studocu.com
Titration Exam Questions ACIDBASE TITRATIONS PAST EXAM QUESTIONS Section 1 Multiple Choice Titration Curve Questions And Answers Pdf Use the information to determine the concentration of the hydrochloric acid. We haven’t added any naoh, so the ph. Figure 1 shows a titration curve. If the acids/bases are strong or weak strong. Titrating a weak acid (hcn) with a strong base (naoh. (2) a steep “equivalence point” region at. Consider the titration of a weak acid, ha, with a. Titration Curve Questions And Answers Pdf.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Questions And Answers Pdf Consider the titration of a weak acid, ha, with a strong base that gives the following ph curve. If the acids/bases are strong or weak strong. Use the information to determine the concentration of the hydrochloric acid. From the graph at the right, determine a. We haven’t added any naoh, so the ph. This question is about acids and bases.. Titration Curve Questions And Answers Pdf.
From www.studypool.com
SOLUTION Titrations questions with answers Studypool Titration Curve Questions And Answers Pdf (1) a fairly flat “buffered” region at low ph; We haven’t added any naoh, so the ph. Titrating a weak acid (hcn) with a strong base (naoh. Volume of titrant (solution in the buret). Titration curve practice problems (key) 1. Use the information to determine the concentration of the hydrochloric acid. Figure 1 shows a titration curve. Practicals for papers. Titration Curve Questions And Answers Pdf.
From www.chegg.com
Solved 1. The titration curve best represents the titration Titration Curve Questions And Answers Pdf Such a curve has three re‐ gions: Consider the titration of a weak acid, ha, with a strong base that gives the following ph curve. We haven’t added any naoh, so the ph. Use the information to determine the concentration of the hydrochloric acid. Titration curve practice problems (key) 1. If the acids/bases are strong or weak strong. Practicals for. Titration Curve Questions And Answers Pdf.
From www.chegg.com
Solved Question 1 At which point in the titration curve Titration Curve Questions And Answers Pdf If the acids/bases are strong or weak strong. (1) a fairly flat “buffered” region at low ph; Figure 1 shows a titration curve. We haven’t added any naoh, so the ph. Titrating a weak acid (hcn) with a strong base (naoh. Practicals for papers 3 and 5. Consider the titration of a weak acid, ha, with a strong base that. Titration Curve Questions And Answers Pdf.
From www.chegg.com
Solved 10. Use the lettered points on the titration curve to Titration Curve Questions And Answers Pdf (2) a steep “equivalence point” region at. Titrating a weak acid (hcn) with a strong base (naoh. Such a curve has three re‐ gions: Titration curve practice problems (key) 1. We haven’t added any naoh, so the ph. A titration curve is a plot of solution ph in a flask vs. If the acids/bases are strong or weak strong. Use. Titration Curve Questions And Answers Pdf.
From www.bartleby.com
Answered At what point on the titration curve… bartleby Titration Curve Questions And Answers Pdf Such a curve has three re‐ gions: If the acids/bases are strong or weak strong. Volume of titrant (solution in the buret). From the graph at the right, determine a. (2) a steep “equivalence point” region at. Practicals for papers 3 and 5. (1) a fairly flat “buffered” region at low ph; Titrating a weak acid (hcn) with a strong. Titration Curve Questions And Answers Pdf.
From www.scribd.com
IGCSE Titration Practice Questions Titration Curve Questions And Answers Pdf Titration curve practice problems (key) 1. If the acids/bases are strong or weak strong. (2) a steep “equivalence point” region at. We haven’t added any naoh, so the ph. Such a curve has three re‐ gions: From the graph at the right, determine a. A titration curve is a plot of solution ph in a flask vs. Practicals for papers. Titration Curve Questions And Answers Pdf.
From www.chegg.com
Solved Refer to the acidbase titration curve shown below. Titration Curve Questions And Answers Pdf Practicals for papers 3 and 5. Use the information to determine the concentration of the hydrochloric acid. (2) a steep “equivalence point” region at. A titration curve is a plot of solution ph in a flask vs. We haven’t added any naoh, so the ph. Figure 1 shows a titration curve. From the graph at the right, determine a. Volume. Titration Curve Questions And Answers Pdf.
From www.solutionspile.com
[Solved] Answer the questions Refer to the titration cu Titration Curve Questions And Answers Pdf We haven’t added any naoh, so the ph. From the graph at the right, determine a. Figure 1 shows a titration curve. If the acids/bases are strong or weak strong. Titration curve practice problems (key) 1. A titration curve is a plot of solution ph in a flask vs. Such a curve has three re‐ gions: Titrating a weak acid. Titration Curve Questions And Answers Pdf.
From www.chegg.com
Solved Refer to the titration curve below. Choose the best Titration Curve Questions And Answers Pdf A titration curve is a plot of solution ph in a flask vs. (2) a steep “equivalence point” region at. Titrating a weak acid (hcn) with a strong base (naoh. Titration curve practice problems (key) 1. Volume of titrant (solution in the buret). This question is about acids and bases. Figure 1 shows a titration curve. Use the information to. Titration Curve Questions And Answers Pdf.
From www.numerade.com
SOLVED Part D Interpreting Titration Curves Using the graphs shown representing the titration Titration Curve Questions And Answers Pdf We haven’t added any naoh, so the ph. Titration curve practice problems (key) 1. (1) a fairly flat “buffered” region at low ph; Titrating a weak acid (hcn) with a strong base (naoh. Use the information to determine the concentration of the hydrochloric acid. Volume of titrant (solution in the buret). From the graph at the right, determine a. Practicals. Titration Curve Questions And Answers Pdf.