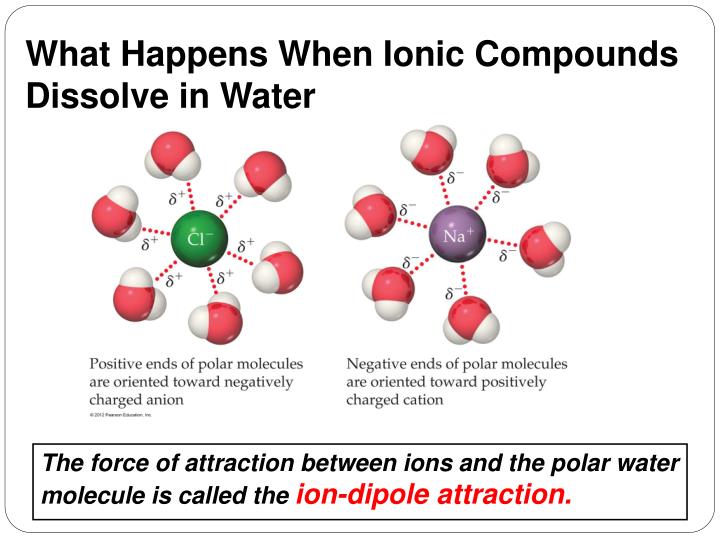
Does Ionic Or Covalent Dissolve Easier In Water . Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. However, when polar covalent molecules dissolve in water, they do not ionize or separate into smaller particles like ionic compounds do. The mechanism by which ionic compounds and covalent compounds. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. We will first examine the process. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. If the attraction between the ions and the water. Water typically dissolves most ionic compounds and polar molecules. Water dissolves many ionic compounds and some covalent compounds. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the.
from www.slideserve.com
The mechanism by which ionic compounds and covalent compounds. However, when polar covalent molecules dissolve in water, they do not ionize or separate into smaller particles like ionic compounds do. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. We will first examine the process. Water dissolves many ionic compounds and some covalent compounds. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. If the attraction between the ions and the water. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation.
PPT UNIT 5 PowerPoint Presentation ID2276550
Does Ionic Or Covalent Dissolve Easier In Water If the attraction between the ions and the water. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The mechanism by which ionic compounds and covalent compounds. We will first examine the process. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. If the attraction between the ions and the water. Water typically dissolves most ionic compounds and polar molecules. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. However, when polar covalent molecules dissolve in water, they do not ionize or separate into smaller particles like ionic compounds do. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. Water dissolves many ionic compounds and some covalent compounds.
From www.studypool.com
SOLUTION Ionic and covalent bonds lab Studypool Does Ionic Or Covalent Dissolve Easier In Water If the attraction between the ions and the water. We will first examine the process. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. The mechanism by which ionic compounds and covalent compounds. Nonpolar molecules, such as those found in grease. Does Ionic Or Covalent Dissolve Easier In Water.
From smallbusinessron.web.fc2.com
do covalent compounds dissolve in water Does Ionic Or Covalent Dissolve Easier In Water If the attraction between the ions and the water. However, when polar covalent molecules dissolve in water, they do not ionize or separate into smaller particles like ionic compounds do. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. When ionic compounds dissolve in water, the ions in. Does Ionic Or Covalent Dissolve Easier In Water.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Does Ionic Or Covalent Dissolve Easier In Water Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. The mechanism by which ionic compounds and covalent compounds. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. We will first examine. Does Ionic Or Covalent Dissolve Easier In Water.
From www.chegg.com
Solved The structure on the far right forms a part of many Does Ionic Or Covalent Dissolve Easier In Water We will first examine the process. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. Nonpolar molecules, such as those. Does Ionic Or Covalent Dissolve Easier In Water.
From slideplayer.com
The Nature of Matter. ppt download Does Ionic Or Covalent Dissolve Easier In Water The mechanism by which ionic compounds and covalent compounds. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. Water dissolves many ionic compounds and some covalent compounds. However, when polar covalent molecules dissolve in water, they do not ionize or separate into smaller particles like ionic. Does Ionic Or Covalent Dissolve Easier In Water.
From slideplayer.com
Polar & nonpolar covalent bonds ppt download Does Ionic Or Covalent Dissolve Easier In Water When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. Water dissolves many ionic compounds and some covalent compounds. However, when polar covalent molecules dissolve in water, they do not ionize or separate into smaller particles like ionic compounds do. If the. Does Ionic Or Covalent Dissolve Easier In Water.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Does Ionic Or Covalent Dissolve Easier In Water When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate. Does Ionic Or Covalent Dissolve Easier In Water.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? in Does Ionic Or Covalent Dissolve Easier In Water Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. However, when polar covalent molecules dissolve in water, they do not ionize or separate into smaller particles like ionic compounds do. When ionic compounds dissolve in water, the. Does Ionic Or Covalent Dissolve Easier In Water.
From slideplayer.com
Types of covalent bonds ppt download Does Ionic Or Covalent Dissolve Easier In Water If the attraction between the ions and the water. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the.. Does Ionic Or Covalent Dissolve Easier In Water.
From sciencing.com
What Happens to Ionic & Covalent Compounds When They Dissolve in Water Does Ionic Or Covalent Dissolve Easier In Water Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. If. Does Ionic Or Covalent Dissolve Easier In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 Does Ionic Or Covalent Dissolve Easier In Water Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. We will first examine the process. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Does Ionic Or Covalent Dissolve Easier In Water.
From www.slideserve.com
PPT Formation of a precipitate PowerPoint Presentation, free download Does Ionic Or Covalent Dissolve Easier In Water When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. If the attraction between the ions and the water. However, when polar covalent molecules dissolve in water, they do not ionize or separate into smaller particles like ionic compounds do. When you. Does Ionic Or Covalent Dissolve Easier In Water.
From www.slideserve.com
PPT Homogeneous and Heterogeneous Mixtures PowerPoint Presentation Does Ionic Or Covalent Dissolve Easier In Water When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water typically dissolves most ionic compounds and polar molecules. However, when polar covalent molecules dissolve in water, they do not ionize or separate into. Does Ionic Or Covalent Dissolve Easier In Water.
From www.myxxgirl.com
How Does Sodium Chloride Nacl Dissolve In Water Vector Image My XXX Does Ionic Or Covalent Dissolve Easier In Water If the attraction between the ions and the water. Water typically dissolves most ionic compounds and polar molecules. We will first examine the process. Water dissolves many ionic compounds and some covalent compounds. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. The mechanism by which. Does Ionic Or Covalent Dissolve Easier In Water.
From meaningkosh.com
Chemical Bond Definition MeaningKosh Does Ionic Or Covalent Dissolve Easier In Water We will first examine the process. However, when polar covalent molecules dissolve in water, they do not ionize or separate into smaller particles like ionic compounds do. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. When ionic compounds are added. Does Ionic Or Covalent Dissolve Easier In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 Does Ionic Or Covalent Dissolve Easier In Water When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. If the attraction between the ions and the water. Water typically dissolves most ionic compounds and polar molecules. We will first examine the process.. Does Ionic Or Covalent Dissolve Easier In Water.
From studycorduanovy.z14.web.core.windows.net
Polarity Of A Molecule Explained Does Ionic Or Covalent Dissolve Easier In Water Water typically dissolves most ionic compounds and polar molecules. The mechanism by which ionic compounds and covalent compounds. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. If the attraction between the ions and the water. When. Does Ionic Or Covalent Dissolve Easier In Water.
From slideplayer.com
1. Which of the following statements about protons is FALSE? ppt download Does Ionic Or Covalent Dissolve Easier In Water Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. Water typically dissolves most ionic compounds and. Does Ionic Or Covalent Dissolve Easier In Water.
From chem.libretexts.org
Solubility and Precipitation Chemistry LibreTexts Does Ionic Or Covalent Dissolve Easier In Water Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. We will first examine the process. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar. Does Ionic Or Covalent Dissolve Easier In Water.
From smallbusinessron.web.fc2.com
do covalent compounds dissolve in water Does Ionic Or Covalent Dissolve Easier In Water Water typically dissolves most ionic compounds and polar molecules. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. Water dissolves many ionic compounds and some covalent compounds. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during. Does Ionic Or Covalent Dissolve Easier In Water.
From www.slideserve.com
PPT The Nature of Aqueous Solutions and Molarity and Solution Does Ionic Or Covalent Dissolve Easier In Water When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. Water typically dissolves most ionic compounds and polar molecules. We will first examine the process. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. Nonpolar. Does Ionic Or Covalent Dissolve Easier In Water.
From www.studocu.com
Copy of Ionic or Covalent Lab Honors 2023 Name Does Ionic Or Covalent Dissolve Easier In Water Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. The mechanism by which ionic compounds and covalent compounds. Water typically dissolves most ionic compounds and polar molecules. When you immerse an ionic compound in water, the ions. Does Ionic Or Covalent Dissolve Easier In Water.
From quizlet.com
Ionic and Covalent Bonds Diagram Quizlet Does Ionic Or Covalent Dissolve Easier In Water The mechanism by which ionic compounds and covalent compounds. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. Water dissolves many ionic compounds and some covalent compounds. We will first examine the process. When ionic compounds are added to water, individual. Does Ionic Or Covalent Dissolve Easier In Water.
From slideplayer.com
Chapter 6 Chemical Reactions Occur in Predictable Ways ppt download Does Ionic Or Covalent Dissolve Easier In Water Water dissolves many ionic compounds and some covalent compounds. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. If the attraction between the ions and the water. The mechanism by which ionic compounds and covalent compounds. Ionic compounds dissolve in water. Does Ionic Or Covalent Dissolve Easier In Water.
From slideplayer.com
Energy Matters Compounds and Bonding ppt download Does Ionic Or Covalent Dissolve Easier In Water We will first examine the process. If the attraction between the ions and the water. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. The mechanism by which ionic compounds and covalent compounds. When ionic compounds dissolve. Does Ionic Or Covalent Dissolve Easier In Water.
From www.studypool.com
SOLUTION Ionic and covalent bond Studypool Does Ionic Or Covalent Dissolve Easier In Water However, when polar covalent molecules dissolve in water, they do not ionize or separate into smaller particles like ionic compounds do. The mechanism by which ionic compounds and covalent compounds. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Does Ionic Or Covalent Dissolve Easier In Water.
From www.chegg.com
Solved 13) Water can dissolve many ionic and polar covalent Does Ionic Or Covalent Dissolve Easier In Water Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. The mechanism by which ionic. Does Ionic Or Covalent Dissolve Easier In Water.
From courses.lumenlearning.com
Reading Covalent Bonds Biology I Does Ionic Or Covalent Dissolve Easier In Water Water typically dissolves most ionic compounds and polar molecules. The mechanism by which ionic compounds and covalent compounds. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. Nonpolar molecules, such as those found in grease or oil, do not dissolve in. Does Ionic Or Covalent Dissolve Easier In Water.
From www.studypool.com
SOLUTION Ionic and covalent bond Studypool Does Ionic Or Covalent Dissolve Easier In Water If the attraction between the ions and the water. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in. Does Ionic Or Covalent Dissolve Easier In Water.
From slideplayer.com
Chemical Bonding I CHEMISTRY 161 Chapter 9 Chemical Bonding I ppt Does Ionic Or Covalent Dissolve Easier In Water When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. We will first examine the process. Water dissolves many ionic compounds and some covalent compounds. When. Does Ionic Or Covalent Dissolve Easier In Water.
From www.studypool.com
SOLUTION Ionic and covalent bond Studypool Does Ionic Or Covalent Dissolve Easier In Water We will first examine the process. Water dissolves many ionic compounds and some covalent compounds. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. Nonpolar molecules, such as those found in grease or oil, do not dissolve. Does Ionic Or Covalent Dissolve Easier In Water.
From pediaa.com
Difference Between Covalent and Ionic Bonds Does Ionic Or Covalent Dissolve Easier In Water The mechanism by which ionic compounds and covalent compounds. When you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. Water dissolves many ionic compounds and some covalent compounds. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for. Does Ionic Or Covalent Dissolve Easier In Water.
From users.highland.edu
Aqueous Solutions Does Ionic Or Covalent Dissolve Easier In Water Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break. Does Ionic Or Covalent Dissolve Easier In Water.
From fun-hindi.com
What Is Ionic Bond Explain With Example Does Ionic Or Covalent Dissolve Easier In Water When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the. The mechanism by which ionic compounds and. Does Ionic Or Covalent Dissolve Easier In Water.
From www.slideserve.com
PPT The Chemical & Physical Basis of Life PowerPoint Presentation Does Ionic Or Covalent Dissolve Easier In Water The mechanism by which ionic compounds and covalent compounds. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Water dissolves many ionic compounds and some covalent compounds. However, when polar covalent molecules dissolve in water, they do not ionize or separate into smaller particles like ionic compounds do. When ionic compounds are added to. Does Ionic Or Covalent Dissolve Easier In Water.