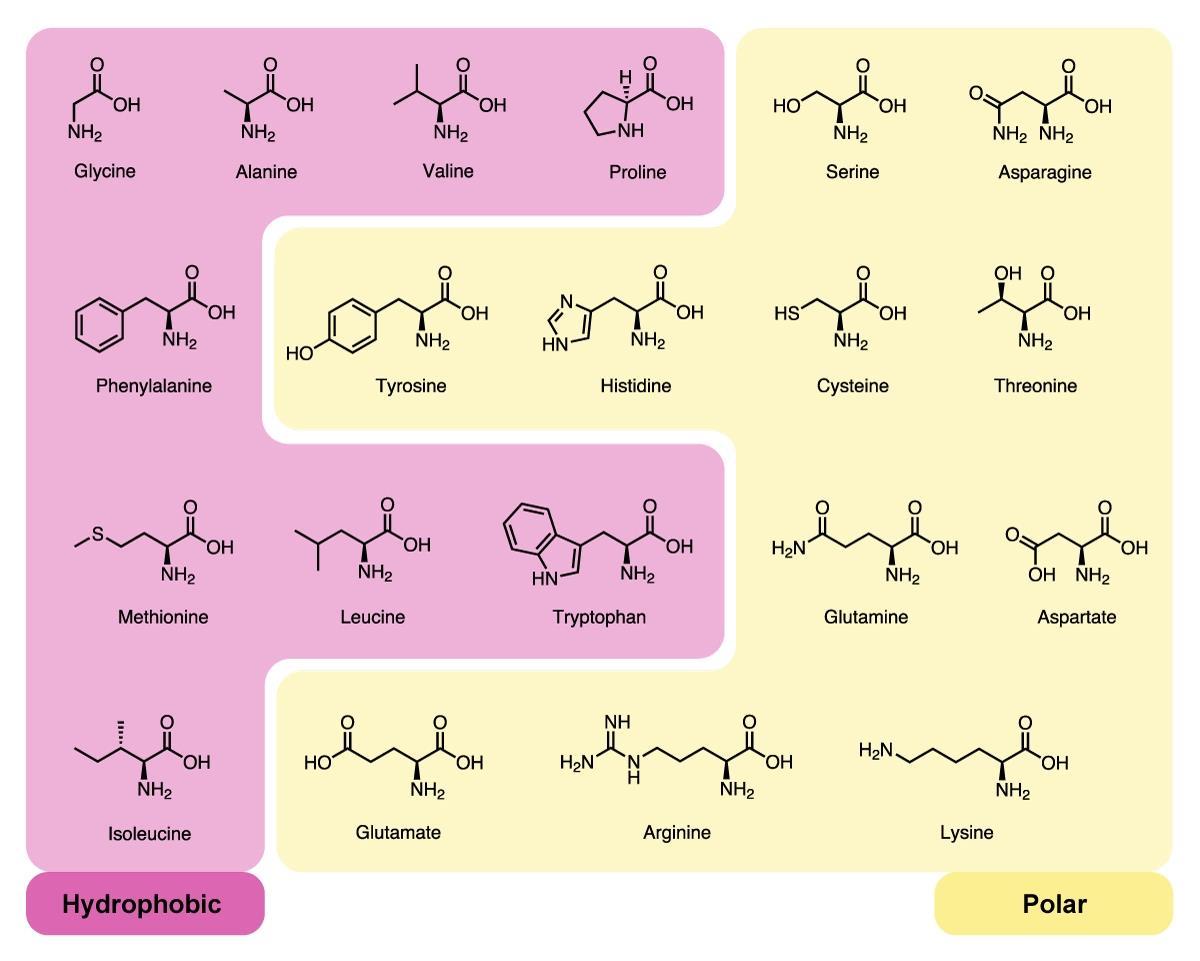
Hydrophobic Amino Acids In Oil . The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. The answer to that question, as well as the positioning of hydrophobic amino acids in the interior of water soluble proteins, is the. Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. A complete understanding of this effect requires the. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. The hydrophobic effect is a major driving force in protein folding.
from www.chemistryworld.com
The answer to that question, as well as the positioning of hydrophobic amino acids in the interior of water soluble proteins, is the. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. The hydrophobic effect is a major driving force in protein folding. The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. A complete understanding of this effect requires the.
Why are there 20 amino acids? Feature Chemistry World
Hydrophobic Amino Acids In Oil Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. A complete understanding of this effect requires the. Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. The hydrophobic effect is a major driving force in protein folding. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. The answer to that question, as well as the positioning of hydrophobic amino acids in the interior of water soluble proteins, is the.
From citylalaf.weebly.com
Hydrophobic amino acids in aqueous solutions citylalaf Hydrophobic Amino Acids In Oil Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. A complete understanding of this effect requires the. Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. The. Hydrophobic Amino Acids In Oil.
From www.researchgate.net
Hydrophobic amino acids within the first six amino acids of the Hydrophobic Amino Acids In Oil A complete understanding of this effect requires the. The hydrophobic effect is a major driving force in protein folding. Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior. Hydrophobic Amino Acids In Oil.
From tripskasap.weebly.com
Hydrophobic amino acids in oil tripskasap Hydrophobic Amino Acids In Oil The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain. Hydrophobic Amino Acids In Oil.
From entreasmemorias.blogspot.com
90 Amazing How Do Hydrophobic Amino Acids React To Oil insectza Hydrophobic Amino Acids In Oil These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. A complete understanding of this effect requires the. Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. The. Hydrophobic Amino Acids In Oil.
From www.chemistryworld.com
Why are there 20 amino acids? Feature Chemistry World Hydrophobic Amino Acids In Oil Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic. Hydrophobic Amino Acids In Oil.
From animalia-life.club
Hydrophilic Examples Hydrophobic Amino Acids In Oil The hydrophobic effect is a major driving force in protein folding. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical. Hydrophobic Amino Acids In Oil.
From lightuniverse.weebly.com
How would a hydrophobic amino acids react to water and oil lightuniverse Hydrophobic Amino Acids In Oil The answer to that question, as well as the positioning of hydrophobic amino acids in the interior of water soluble proteins, is the. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. A complete understanding of this effect requires the. These amino acids exhibited amphipathic nature due to their. Hydrophobic Amino Acids In Oil.
From exowgjrmc.blob.core.windows.net
Amino Acids Hydrophobic Hydrophilic at Leticia Ridley blog Hydrophobic Amino Acids In Oil The answer to that question, as well as the positioning of hydrophobic amino acids in the interior of water soluble proteins, is the. Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. The. Hydrophobic Amino Acids In Oil.
From caqweblind.weebly.com
Properties of hydrophobic amino acids caqweblind Hydrophobic Amino Acids In Oil Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. A complete understanding of this effect requires the. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and. Hydrophobic Amino Acids In Oil.
From www.researchgate.net
The relevance of the basic and hydrophobic amino acids constituting the Hydrophobic Amino Acids In Oil The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain. Hydrophobic Amino Acids In Oil.
From copperascse.weebly.com
Hydrophobic amino acids with nonpolar side chains copperascse Hydrophobic Amino Acids In Oil The hydrophobic effect is a major driving force in protein folding. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. The answer to that question, as well as the positioning of hydrophobic amino acids in the. Hydrophobic Amino Acids In Oil.
From levnepneu-online.cz
Jaký je rozdíl mezi hydrofobem a hydrofilem rozumíme základním pojmům Hydrophobic Amino Acids In Oil The hydrophobic effect is a major driving force in protein folding. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. Hydrophobic interactions are involved in and. Hydrophobic Amino Acids In Oil.
From lassafood.weebly.com
Family hydrophobic amino acids lassafood Hydrophobic Amino Acids In Oil Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. The hydrophobic effect is a major driving force in protein folding. Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and. Hydrophobic Amino Acids In Oil.
From www.semanticscholar.org
Figure 2 from The use of hydrophobic amino acids in protecting spray Hydrophobic Amino Acids In Oil The hydrophobic effect is a major driving force in protein folding. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. A complete understanding of this effect requires the. Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. The answer to. Hydrophobic Amino Acids In Oil.
From houndopec.weebly.com
Do hydrophobic amino acids have nonpolar side chains houndopec Hydrophobic Amino Acids In Oil These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just. Hydrophobic Amino Acids In Oil.
From ar.inspiredpencil.com
Hydrophobic And Hydrophilic Amino Acids Hydrophobic Amino Acids In Oil Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. The hydrophobic effect is a major driving force in protein folding. A complete understanding of this effect requires the. The answer to that question, as. Hydrophobic Amino Acids In Oil.
From slides.com
HP Protein Folding is Hydrophobic Amino Acids In Oil Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. Hydrophobic interactions are involved in and believed to be the fundamental driving. Hydrophobic Amino Acids In Oil.
From www.semanticscholar.org
Figure 8 from The use of hydrophobic amino acids in protecting spray Hydrophobic Amino Acids In Oil The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. A complete understanding of this effect requires the. The answer to that. Hydrophobic Amino Acids In Oil.
From www.researchgate.net
FIGURE The computed parameters of αSScasein; (A) hydrophobic amino Hydrophobic Amino Acids In Oil The answer to that question, as well as the positioning of hydrophobic amino acids in the interior of water soluble proteins, is the. A complete understanding of this effect requires the. The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. Hydrophobic. Hydrophobic Amino Acids In Oil.
From www.pinterest.com
TJ. Hydrophobic Amino Acids. Amino acids are grouped according to what Hydrophobic Amino Acids In Oil Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. The answer to that question, as well as the positioning of hydrophobic amino acids in the interior of water soluble proteins, is. Hydrophobic Amino Acids In Oil.
From www.semanticscholar.org
Figure 6 from The use of hydrophobic amino acids in protecting spray Hydrophobic Amino Acids In Oil Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. A complete understanding of this effect requires the. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. The hydrophobic effect is a major driving force in protein folding. Emulsions are also formed in the presence of. Hydrophobic Amino Acids In Oil.
From www.slideserve.com
PPT From Sequences to Structure PowerPoint Presentation, free Hydrophobic Amino Acids In Oil Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. The hydrophobic effect is a major driving force in protein folding. A complete understanding of this effect requires the. The answer to that question, as well as the positioning of hydrophobic amino acids in the interior of water soluble proteins,. Hydrophobic Amino Acids In Oil.
From exocringu.blob.core.windows.net
What Chemical Is Hydrophobic at Mary Barden blog Hydrophobic Amino Acids In Oil Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. The answer to that question, as well as the positioning of hydrophobic amino acids in the interior of water soluble proteins, is the. A complete understanding of this effect requires the. The hydrophobic effect is a major driving force in protein folding.. Hydrophobic Amino Acids In Oil.
From schoolbag.info
Biologically Important Molecules MCAT Biology and Biochemistry Hydrophobic Amino Acids In Oil These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. The nonpolar (hydrophobic) side chains in a. Hydrophobic Amino Acids In Oil.
From wisc.pb.unizin.org
D26.2 Amino Acids Chemistry 109 Fall 2021 Hydrophobic Amino Acids In Oil The answer to that question, as well as the positioning of hydrophobic amino acids in the interior of water soluble proteins, is the. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. Emulsions are also formed. Hydrophobic Amino Acids In Oil.
From sublockq.weebly.com
Hydrophobic amino acids in oil sublockq Hydrophobic Amino Acids In Oil A complete understanding of this effect requires the. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged. Hydrophobic Amino Acids In Oil.
From www.researchgate.net
Hydrophobic amino acids that are interspersed with acidic, serine Hydrophobic Amino Acids In Oil These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. The hydrophobic effect is a major driving force in protein folding. Hydrophobic interactions are involved in and. Hydrophobic Amino Acids In Oil.
From indianavsera.weebly.com
Hydrophobic amino acids with nonpolar side chains indianavsera Hydrophobic Amino Acids In Oil Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic. Hydrophobic Amino Acids In Oil.
From ar.inspiredpencil.com
Hydrophobic And Hydrophilic Amino Acids Hydrophobic Amino Acids In Oil Hydrophobic interactions are involved in and believed to be the fundamental driving force of many chemical and biological. A complete understanding of this effect requires the. These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the. Hydrophobic Amino Acids In Oil.
From www.semanticscholar.org
Figure 1 from The use of hydrophobic amino acids in protecting spray Hydrophobic Amino Acids In Oil The answer to that question, as well as the positioning of hydrophobic amino acids in the interior of water soluble proteins, is the. Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the. Hydrophobic Amino Acids In Oil.
From www.dummies.com
Nonpolar (Hydrophobic) Amino Acids of Biochemistry dummies Hydrophobic Amino Acids In Oil Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. The hydrophobic effect is a major driving force in protein folding. Hydrophobic interactions are. Hydrophobic Amino Acids In Oil.
From nowdax.weebly.com
How to tell hydrophobic amino acids nowdax Hydrophobic Amino Acids In Oil These amino acids exhibited amphipathic nature due to their hydrophobic alkyl chain and charged head. Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. The answer to that question, as well as the positioning of hydrophobic amino acids in the interior of water soluble proteins, is the. The hydrophobic effect is. Hydrophobic Amino Acids In Oil.
From eureka.patsnap.com
Hydrophobic oilcontrol powder treated by amino acid and having soft Hydrophobic Amino Acids In Oil The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. The hydrophobic effect is a major driving force in protein folding. Emulsions are also formed in the presence of an emulsifier, which comprises of both hydrophobic and hydrophilic components. Most of the. Hydrophobic Amino Acids In Oil.
From testbook.com
Learn Difference Between Hydrophobic and Hydrophilic Amino Acids Hydrophobic Amino Acids In Oil A complete understanding of this effect requires the. The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in the interior of the molecule (just as. The hydrophobic effect is a major driving force in protein folding. Most of the hydrophilic amino acid groups are at the exterior, while the. Hydrophobic Amino Acids In Oil.
From www.researchgate.net
Mutation of I696 to hydrophobic amino acids affects voltage sensing and Hydrophobic Amino Acids In Oil The hydrophobic effect is a major driving force in protein folding. Most of the hydrophilic amino acid groups are at the exterior, while the hydrophobic residues are located at the interior. A complete understanding of this effect requires the. The nonpolar (hydrophobic) side chains in a protein—belonging to such amino acids as phenylalanine, leucine, valine, and tryptophan—tend to cluster in. Hydrophobic Amino Acids In Oil.