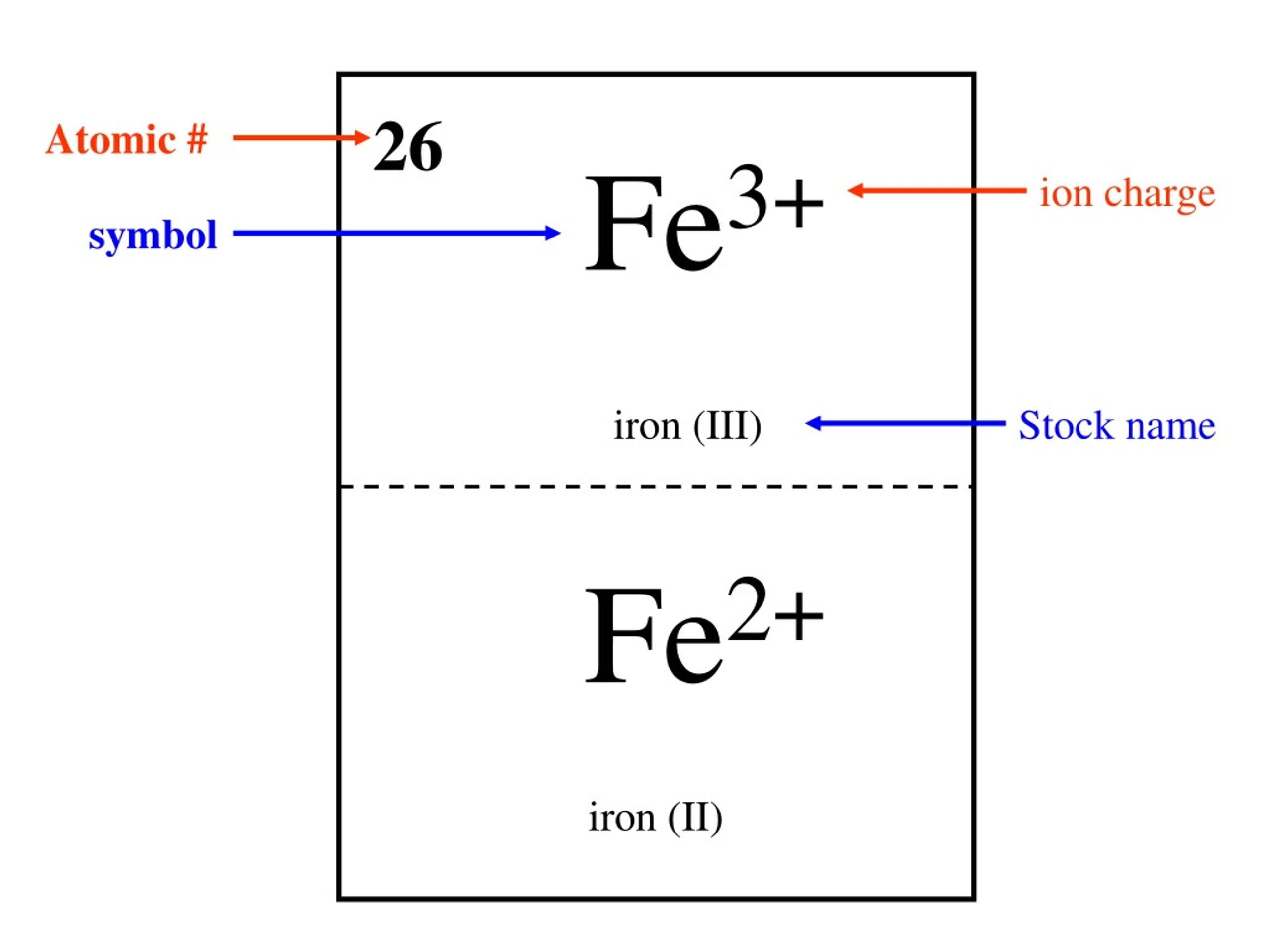
Iron Common Ionic Charge . when there is more than one common ion, the more common ion is shown on top. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce. This table contains the most common charges along with the element. Elements for which no ion is shown are. most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. 93 rows — — looking for a periodic table of elements with common charges? As an example, iron commonly forms two different ions. — based on the common charges, we know iron can lose two or three electrons to achieve a stable electron configuration, resulting in.
from exowpbmls.blob.core.windows.net
It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce. As an example, iron commonly forms two different ions. Elements for which no ion is shown are. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. when there is more than one common ion, the more common ion is shown on top. This table contains the most common charges along with the element. most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. 93 rows — — looking for a periodic table of elements with common charges? — based on the common charges, we know iron can lose two or three electrons to achieve a stable electron configuration, resulting in.
Charge Of Iron In Fe2O3 at Belinda Escudero blog
Iron Common Ionic Charge As an example, iron commonly forms two different ions. This table contains the most common charges along with the element. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. 93 rows — — looking for a periodic table of elements with common charges? Elements for which no ion is shown are. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce. when there is more than one common ion, the more common ion is shown on top. — based on the common charges, we know iron can lose two or three electrons to achieve a stable electron configuration, resulting in. As an example, iron commonly forms two different ions.
From www.flinnsci.ca
Ion Names, Formulas, and Charges Charts for Chemistry Iron Common Ionic Charge Elements for which no ion is shown are. This table contains the most common charges along with the element. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. As an example, iron commonly forms two different ions. when there is more than one common ion, the more common. Iron Common Ionic Charge.
From exowpbmls.blob.core.windows.net
Charge Of Iron In Fe2O3 at Belinda Escudero blog Iron Common Ionic Charge 93 rows — — looking for a periodic table of elements with common charges? Elements for which no ion is shown are. — based on the common charges, we know iron can lose two or three electrons to achieve a stable electron configuration, resulting in. most transition metals differ from the metals of groups 1, 2, and. Iron Common Ionic Charge.
From chem.libretexts.org
Chapter 4.1 Ionic Bonding Chemistry LibreTexts Iron Common Ionic Charge — based on the common charges, we know iron can lose two or three electrons to achieve a stable electron configuration, resulting in. Elements for which no ion is shown are. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce. This table contains the most. Iron Common Ionic Charge.
From utedzz.blogspot.com
Periodic Table With Common Ionic Charges Periodic Table Timeline Iron Common Ionic Charge It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce. most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. This table contains the most common charges along. Iron Common Ionic Charge.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation, free download ID Iron Common Ionic Charge It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce. This table contains the most common charges along with the element. when there is more than one common ion, the more common ion is shown on top. — based on the common charges, we know. Iron Common Ionic Charge.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Iron Common Ionic Charge when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. Elements for which no ion is shown are. This table contains the most common charges along with the element. — based on the common charges, we know iron can lose two or three electrons to achieve a stable electron. Iron Common Ionic Charge.
From lessonlibrarysummered.z13.web.core.windows.net
How To Determine Charges Of An Ion Iron Common Ionic Charge when there is more than one common ion, the more common ion is shown on top. Elements for which no ion is shown are. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. 93 rows — — looking for a periodic table of elements with common charges?. Iron Common Ionic Charge.
From correo.muycomputer.com
Determining The Ionic Charge Worksheet Printable Kids Entertainment Iron Common Ionic Charge when there is more than one common ion, the more common ion is shown on top. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. 93 rows — — looking for a periodic table of elements with common charges? It can sometimes lose two electrons to form. Iron Common Ionic Charge.
From elchoroukhost.net
Periodic Table With Charges Of Ions Elcho Table Iron Common Ionic Charge when there is more than one common ion, the more common ion is shown on top. most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. This table contains the most common charges along with the element. when naming. Iron Common Ionic Charge.
From learningcampusvalverde.z21.web.core.windows.net
List Of Ions And Charges Iron Common Ionic Charge Elements for which no ion is shown are. This table contains the most common charges along with the element. 93 rows — — looking for a periodic table of elements with common charges? As an example, iron commonly forms two different ions. — based on the common charges, we know iron can lose two or three electrons to. Iron Common Ionic Charge.
From www.pinterest.com
A Guide to Common Polyatomic Ions Colour Version Polyatomic ion Iron Common Ionic Charge 93 rows — — looking for a periodic table of elements with common charges? Elements for which no ion is shown are. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses. Iron Common Ionic Charge.
From studylib.net
Ionic charges chart Iron Common Ionic Charge when there is more than one common ion, the more common ion is shown on top. Elements for which no ion is shown are. As an example, iron commonly forms two different ions. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. It can sometimes lose two electrons. Iron Common Ionic Charge.
From classfullredrafts.z13.web.core.windows.net
How To Identify Charges Of Ions Iron Common Ionic Charge This table contains the most common charges along with the element. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges.. Iron Common Ionic Charge.
From utedzz.blogspot.com
Common Ion Charges Periodic Table Periodic Table Timeline Iron Common Ionic Charge It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce. when there is more than one common ion, the more common ion is shown on top. This table contains the most common charges along with the element. most transition metals differ from the metals of. Iron Common Ionic Charge.
From www.youtube.com
Valence Electrons & Ionic Charge and the Periodic Table YouTube Iron Common Ionic Charge Elements for which no ion is shown are. — based on the common charges, we know iron can lose two or three electrons to achieve a stable electron configuration, resulting in. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. most transition metals differ from the metals. Iron Common Ionic Charge.
From en.wikipedia.org
Ionic bonding Wikipedia Iron Common Ionic Charge As an example, iron commonly forms two different ions. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. when there is more than one common ion, the more common ion is shown on top. most transition metals differ from the metals of groups 1, 2, and 13. Iron Common Ionic Charge.
From elchoroukhost.net
Periodic Table With Ionic Charges And Names Elcho Table Iron Common Ionic Charge This table contains the most common charges along with the element. Elements for which no ion is shown are. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce. As an example, iron commonly forms two different ions. most transition metals differ from the metals of. Iron Common Ionic Charge.
From scientifictutor.org
Chem Ions Scientific Tutor Iron Common Ionic Charge Elements for which no ion is shown are. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. — based on the common charges, we know. Iron Common Ionic Charge.
From sciencenotes.org
Atomic Radius and Ionic Radius Iron Common Ionic Charge As an example, iron commonly forms two different ions. when there is more than one common ion, the more common ion is shown on top. This table contains the most common charges along with the element. Elements for which no ion is shown are. most transition metals differ from the metals of groups 1, 2, and 13 in. Iron Common Ionic Charge.
From sciencetrends.com
Periodic Table Ionic Charges, Name, And Mass Science Trends Iron Common Ionic Charge 93 rows — — looking for a periodic table of elements with common charges? As an example, iron commonly forms two different ions. most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. when naming ionic compounds whose cations. Iron Common Ionic Charge.
From utedzz.blogspot.com
Common Ion Charges Periodic Table Periodic Table Timeline Iron Common Ionic Charge It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce. This table contains the most common charges along with the element. As an example, iron commonly forms two different ions. Elements for which no ion is shown are. when there is more than one common ion,. Iron Common Ionic Charge.
From www.nemoquiz.com
Ion Charge from Periodic Table NemoQuiz Iron Common Ionic Charge Elements for which no ion is shown are. when there is more than one common ion, the more common ion is shown on top. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. — based on the common charges, we know iron can lose two or three. Iron Common Ionic Charge.
From www.sliderbase.com
Ionic Compound Nomenclature Presentation Chemistry Iron Common Ionic Charge This table contains the most common charges along with the element. — based on the common charges, we know iron can lose two or three electrons to achieve a stable electron configuration, resulting in. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. It can sometimes lose two. Iron Common Ionic Charge.
From elchoroukhost.net
Periodic Table With Ionic Charges Printable Elcho Table Iron Common Ionic Charge It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. Elements for which no ion is shown are. This table contains the most common charges along with. Iron Common Ionic Charge.
From sciencenotes.org
Periodic Table Charge Blk BG Science Notes and Projects Iron Common Ionic Charge — based on the common charges, we know iron can lose two or three electrons to achieve a stable electron configuration, resulting in. 93 rows — — looking for a periodic table of elements with common charges? As an example, iron commonly forms two different ions. Elements for which no ion is shown are. This table contains the. Iron Common Ionic Charge.
From www.youtube.com
Ion Charges GCSE Chemistry Revision YouTube Iron Common Ionic Charge 93 rows — — looking for a periodic table of elements with common charges? when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. Elements for which no ion is shown are. — based on the common charges, we know iron can lose two or three electrons to. Iron Common Ionic Charge.
From www.slideserve.com
PPT Ionic Compounds Containing Multivalent Ions PowerPoint Iron Common Ionic Charge most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. when there is more than one common ion, the more common ion is shown on top. when naming ionic compounds whose cations can have more than one possible charge,. Iron Common Ionic Charge.
From ar.inspiredpencil.com
Periodic Table With Charges Of Ions Iron Common Ionic Charge when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion,. Iron Common Ionic Charge.
From courses.lumenlearning.com
Molecular and Ionic Compounds Chemistry Iron Common Ionic Charge when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion, while at other times it loses three electrons to form the \(\ce. when there is more than one common ion, the more common ion is shown on top.. Iron Common Ionic Charge.
From www.youtube.com
Finding the Ionic Charge for Elements on the Periodic Table YouTube Iron Common Ionic Charge As an example, iron commonly forms two different ions. 93 rows — — looking for a periodic table of elements with common charges? Elements for which no ion is shown are. when there is more than one common ion, the more common ion is shown on top. It can sometimes lose two electrons to form the \(\ce{fe^{2+}}\) ion,. Iron Common Ionic Charge.
From general.chemistrysteps.com
Naming Ionic Compounds Chemistry Steps Iron Common Ionic Charge most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. — based on the common charges, we know iron can lose two or three electrons to achieve a stable electron configuration, resulting in. when there is more than one. Iron Common Ionic Charge.
From www.thoughtco.com
Periodic Table With Common Ionic Charges Iron Common Ionic Charge 93 rows — — looking for a periodic table of elements with common charges? — based on the common charges, we know iron can lose two or three electrons to achieve a stable electron configuration, resulting in. Elements for which no ion is shown are. As an example, iron commonly forms two different ions. most transition metals. Iron Common Ionic Charge.
From classfullredrafts.z13.web.core.windows.net
Chemistry Charges Of Ions Iron Common Ionic Charge when there is more than one common ion, the more common ion is shown on top. This table contains the most common charges along with the element. 93 rows — — looking for a periodic table of elements with common charges? most transition metals differ from the metals of groups 1, 2, and 13 in that they. Iron Common Ionic Charge.
From www.youtube.com
Ionic Charge for Iron (Fe) YouTube Iron Common Ionic Charge — based on the common charges, we know iron can lose two or three electrons to achieve a stable electron configuration, resulting in. when naming ionic compounds whose cations can have more than one possible charge, we must also include the charge,. This table contains the most common charges along with the element. It can sometimes lose two. Iron Common Ionic Charge.
From sciencenotes.org
Printable Periodic Table Element Charges Iron Common Ionic Charge 93 rows — — looking for a periodic table of elements with common charges? most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. This table contains the most common charges along with the element. Elements for which no ion. Iron Common Ionic Charge.