Enzyme Binding Constant . What is charged is the rate at which equilibrium is. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. Enzymes have varying tendencies to bind their substrates ( affinities ). kon is the association rate constant, [p] is the concentration of. E + s−→k1 [es]−→k2 e + p (1) (1) e +. enzymes do not affect the thermodynamics of reactions. from the constancy of the binding curves in figure 6b, we can conclude that the binding. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged.
from www.expii.com
from the constancy of the binding curves in figure 6b, we can conclude that the binding. What is charged is the rate at which equilibrium is. Enzymes have varying tendencies to bind their substrates ( affinities ). E + s−→k1 [es]−→k2 e + p (1) (1) e +. enzymes do not affect the thermodynamics of reactions. kon is the association rate constant, [p] is the concentration of. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged.
Enzyme Inhibition — Overview & Types Expii
Enzyme Binding Constant Enzymes have varying tendencies to bind their substrates ( affinities ). E + s−→k1 [es]−→k2 e + p (1) (1) e +. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. from the constancy of the binding curves in figure 6b, we can conclude that the binding. Enzymes have varying tendencies to bind their substrates ( affinities ). What is charged is the rate at which equilibrium is. enzymes do not affect the thermodynamics of reactions. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. kon is the association rate constant, [p] is the concentration of.
From www.slideserve.com
PPT Allosteric enzymes PowerPoint Presentation ID3759029 Enzyme Binding Constant E + s−→k1 [es]−→k2 e + p (1) (1) e +. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. enzymes do not affect the thermodynamics of reactions. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. What is charged is the rate at which equilibrium. Enzyme Binding Constant.
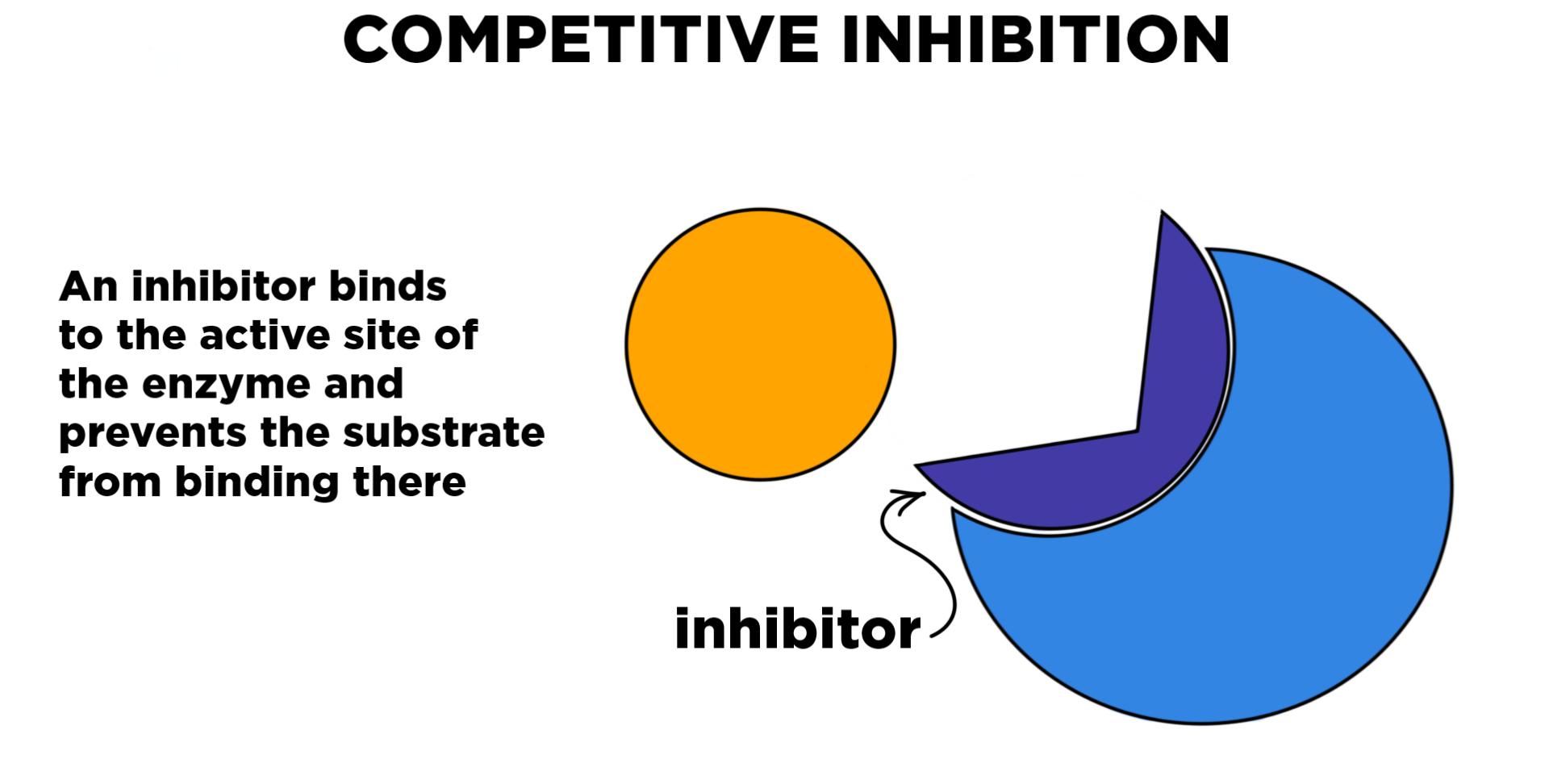
From abmeia.blogspot.com
Understanding Enzyme saturation curve Enzyme Binding Constant kon is the association rate constant, [p] is the concentration of. from the constancy of the binding curves in figure 6b, we can conclude that the binding. enzymes do not affect the thermodynamics of reactions. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. E + s−→k1 [es]−→k2 e + p (1) (1). Enzyme Binding Constant.
From www.nagwa.com
Question Video Understanding How the Substrate Binds to an Enzyme Nagwa Enzyme Binding Constant Enzymes have varying tendencies to bind their substrates ( affinities ). kon is the association rate constant, [p] is the concentration of. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. What is charged is the rate. Enzyme Binding Constant.
From www.researchgate.net
EnzymeSubstrate Binding as an Analog Circuit Schematic The Enzyme Binding Constant What is charged is the rate at which equilibrium is. E + s−→k1 [es]−→k2 e + p (1) (1) e +. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. kon is the association rate constant, [p] is the concentration of. enzymes do not affect the thermodynamics of reactions.. Enzyme Binding Constant.
From www.researchgate.net
Measurements of binding affinity and enzyme Measurements of Enzyme Binding Constant Enzymes have varying tendencies to bind their substrates ( affinities ). from the constancy of the binding curves in figure 6b, we can conclude that the binding. kon is the association rate constant, [p] is the concentration of. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. E +. Enzyme Binding Constant.
From fity.club
Enzyme Enzyme Binding Constant An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. E + s−→k1 [es]−→k2 e + p (1) (1) e +. What is charged is the rate at which equilibrium is. kon is the association rate constant, [p] is the concentration of. For reversible reactions (as an example), the equilibrium constant,. Enzyme Binding Constant.
From bio.libretexts.org
3.4 Regulation of Enzyme Activity Biology LibreTexts Enzyme Binding Constant E + s−→k1 [es]−→k2 e + p (1) (1) e +. Enzymes have varying tendencies to bind their substrates ( affinities ). from the constancy of the binding curves in figure 6b, we can conclude that the binding. kon is the association rate constant, [p] is the concentration of. enzymes do not affect the thermodynamics of reactions.. Enzyme Binding Constant.
From www.slideserve.com
PPT Lecture 12 Enzyme Catalysis PowerPoint Presentation, free Enzyme Binding Constant What is charged is the rate at which equilibrium is. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. E + s−→k1 [es]−→k2 e + p (1) (1) e +. kon is the association rate constant, [p] is the concentration of. Enzymes have varying tendencies to bind their substrates (. Enzyme Binding Constant.
From www.slideserve.com
PPT Chapter 6.3 Enzyme PowerPoint Presentation, free Enzyme Binding Constant from the constancy of the binding curves in figure 6b, we can conclude that the binding. E + s−→k1 [es]−→k2 e + p (1) (1) e +. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. . Enzyme Binding Constant.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Enzyme Binding Constant from the constancy of the binding curves in figure 6b, we can conclude that the binding. What is charged is the rate at which equilibrium is. Enzymes have varying tendencies to bind their substrates ( affinities ). kon is the association rate constant, [p] is the concentration of. For reversible reactions (as an example), the equilibrium constant, k. Enzyme Binding Constant.
From courses.lumenlearning.com
18.6 Enzyme Action The Basics of General, Organic, and Biological Enzyme Binding Constant Enzymes have varying tendencies to bind their substrates ( affinities ). kon is the association rate constant, [p] is the concentration of. What is charged is the rate at which equilibrium is. from the constancy of the binding curves in figure 6b, we can conclude that the binding. An enzyme's k m describes the substrate concentration at which. Enzyme Binding Constant.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Enzyme Binding Constant Enzymes have varying tendencies to bind their substrates ( affinities ). E + s−→k1 [es]−→k2 e + p (1) (1) e +. enzymes do not affect the thermodynamics of reactions. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. from the constancy of the binding curves in figure 6b, we can conclude that the. Enzyme Binding Constant.
From what-when-how.com
SlowBinding Enzyme Inhibition (Molecular Biology) Enzyme Binding Constant kon is the association rate constant, [p] is the concentration of. E + s−→k1 [es]−→k2 e + p (1) (1) e +. What is charged is the rate at which equilibrium is. Enzymes have varying tendencies to bind their substrates ( affinities ). An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are. Enzyme Binding Constant.
From www.savemyexams.co.uk
Enzyme Activity Substrate Concentration (2.4.6) OCR A Level Biology Enzyme Binding Constant For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. Enzymes have varying tendencies to bind their substrates ( affinities ). from the constancy of the binding curves in figure 6b, we can conclude that the binding. What is charged is the rate at which equilibrium is. An enzyme's k m describes the substrate concentration at. Enzyme Binding Constant.
From courses.lumenlearning.com
Enzymes OpenStax Biology 2e Enzyme Binding Constant enzymes do not affect the thermodynamics of reactions. What is charged is the rate at which equilibrium is. kon is the association rate constant, [p] is the concentration of. E + s−→k1 [es]−→k2 e + p (1) (1) e +. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by.. Enzyme Binding Constant.
From www.slideserve.com
PPT Chapter 6 Enzymes PowerPoint Presentation, free download ID5143485 Enzyme Binding Constant What is charged is the rate at which equilibrium is. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. E + s−→k1 [es]−→k2 e + p (1) (1) e +. kon is the association rate constant, [p] is the concentration of. For reversible reactions (as an example), the equilibrium constant,. Enzyme Binding Constant.
From fity.club
Enzyme Enzyme Binding Constant Enzymes have varying tendencies to bind their substrates ( affinities ). E + s−→k1 [es]−→k2 e + p (1) (1) e +. from the constancy of the binding curves in figure 6b, we can conclude that the binding. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. What is charged is the rate at which. Enzyme Binding Constant.
From www.biologyexams4u.com
Biology Exams 4 U Enzyme Binding Constant Enzymes have varying tendencies to bind their substrates ( affinities ). What is charged is the rate at which equilibrium is. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. from the constancy of the binding curves in figure 6b, we can conclude that the binding. kon is the. Enzyme Binding Constant.
From science.halleyhosting.com
Chapter 8 Enzymes Enzyme Binding Constant What is charged is the rate at which equilibrium is. from the constancy of the binding curves in figure 6b, we can conclude that the binding. Enzymes have varying tendencies to bind their substrates ( affinities ). An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. kon is the. Enzyme Binding Constant.
From www.youtube.com
Lecture 4C EnzymeSubstrate Binding YouTube Enzyme Binding Constant enzymes do not affect the thermodynamics of reactions. kon is the association rate constant, [p] is the concentration of. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. What is charged is the rate at which equilibrium is. from the constancy of the binding curves in figure 6b,. Enzyme Binding Constant.
From www.expii.com
Enzyme Inhibition — Overview & Types Expii Enzyme Binding Constant What is charged is the rate at which equilibrium is. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. E + s−→k1 [es]−→k2 e + p (1) (1) e +. enzymes do not affect the thermodynamics of reactions. from the constancy of the binding curves in figure 6b, we. Enzyme Binding Constant.
From microbenotes.com
Enzymes Properties, Classification and Significance Enzyme Binding Constant Enzymes have varying tendencies to bind their substrates ( affinities ). E + s−→k1 [es]−→k2 e + p (1) (1) e +. enzymes do not affect the thermodynamics of reactions. kon is the association rate constant, [p] is the concentration of. from the constancy of the binding curves in figure 6b, we can conclude that the binding.. Enzyme Binding Constant.
From open.lib.umn.edu
13. EnzymeLinked Receptors Principles of Pharmacology Study Guide Enzyme Binding Constant kon is the association rate constant, [p] is the concentration of. What is charged is the rate at which equilibrium is. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. enzymes do not affect the thermodynamics of reactions. For reversible reactions (as an example), the equilibrium constant, k eq,. Enzyme Binding Constant.
From www.slideserve.com
PPT Chapter 6 Enzyme PowerPoint Presentation, free download ID5692414 Enzyme Binding Constant enzymes do not affect the thermodynamics of reactions. from the constancy of the binding curves in figure 6b, we can conclude that the binding. What is charged is the rate at which equilibrium is. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. For reversible reactions (as an example),. Enzyme Binding Constant.
From elifesciences.org
How to measure and evaluate binding affinities eLife Enzyme Binding Constant For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. Enzymes have varying tendencies to bind their substrates ( affinities ). enzymes do not affect the thermodynamics of reactions. kon is the association rate constant, [p] is the concentration of. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites. Enzyme Binding Constant.
From www.sciencefacts.net
Enzyme Definition, Types, Structure, Functions, & Diagram Enzyme Binding Constant E + s−→k1 [es]−→k2 e + p (1) (1) e +. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. enzymes do not affect the thermodynamics of reactions. What is charged is the rate at which equilibrium is. Enzymes have varying tendencies to bind their substrates ( affinities ). kon is the association rate. Enzyme Binding Constant.
From bio.libretexts.org
4.10 Enzyme Inhibition Biology LibreTexts Enzyme Binding Constant E + s−→k1 [es]−→k2 e + p (1) (1) e +. enzymes do not affect the thermodynamics of reactions. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. Enzymes have varying tendencies to bind their substrates ( affinities ). from the constancy of the binding curves in figure 6b,. Enzyme Binding Constant.
From bio.libretexts.org
1.1 Cellular Foundations Biology LibreTexts Enzyme Binding Constant E + s−→k1 [es]−→k2 e + p (1) (1) e +. enzymes do not affect the thermodynamics of reactions. What is charged is the rate at which equilibrium is. kon is the association rate constant, [p] is the concentration of. from the constancy of the binding curves in figure 6b, we can conclude that the binding. Enzymes. Enzyme Binding Constant.
From www.researchgate.net
Binding affinity versus enzyme activity. The enzyme activity of active Enzyme Binding Constant E + s−→k1 [es]−→k2 e + p (1) (1) e +. enzymes do not affect the thermodynamics of reactions. What is charged is the rate at which equilibrium is. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. For reversible reactions (as an example), the equilibrium constant, k eq, is. Enzyme Binding Constant.
From www.expii.com
Enzyme Inhibition — Overview & Types Expii Enzyme Binding Constant What is charged is the rate at which equilibrium is. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. kon is the association rate constant, [p] is the concentration of. Enzymes have varying tendencies to bind their. Enzyme Binding Constant.
From www.slideserve.com
PPT Lecture 6 Measuring enzyme activity PowerPoint Presentation Enzyme Binding Constant enzymes do not affect the thermodynamics of reactions. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. Enzymes have varying tendencies to bind their substrates ( affinities ). from the constancy of the binding curves in figure 6b, we can conclude that the binding. For reversible reactions (as an. Enzyme Binding Constant.
From faculty.samford.edu
Cell Chemistry Enzyme Binding Constant Enzymes have varying tendencies to bind their substrates ( affinities ). from the constancy of the binding curves in figure 6b, we can conclude that the binding. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. What is charged is the rate at which equilibrium is. For reversible reactions (as. Enzyme Binding Constant.
From www.chegg.com
Solved Drugs that bind to enzymes are typically made up of Enzyme Binding Constant What is charged is the rate at which equilibrium is. Enzymes have varying tendencies to bind their substrates ( affinities ). from the constancy of the binding curves in figure 6b, we can conclude that the binding. For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. E + s−→k1 [es]−→k2 e + p (1) (1). Enzyme Binding Constant.
From www.albert.io
Enzymes AP® Biology Crash Course Review Albert.io Enzyme Binding Constant Enzymes have varying tendencies to bind their substrates ( affinities ). For reversible reactions (as an example), the equilibrium constant, k eq, is unchanged. What is charged is the rate at which equilibrium is. from the constancy of the binding curves in figure 6b, we can conclude that the binding. An enzyme's k m describes the substrate concentration at. Enzyme Binding Constant.
From www.researchgate.net
Which software can be used to determine dissociation constant for Enzyme Binding Constant What is charged is the rate at which equilibrium is. An enzyme's k m describes the substrate concentration at which half the enzyme's active sites are occupied by. Enzymes have varying tendencies to bind their substrates ( affinities ). E + s−→k1 [es]−→k2 e + p (1) (1) e +. from the constancy of the binding curves in figure. Enzyme Binding Constant.