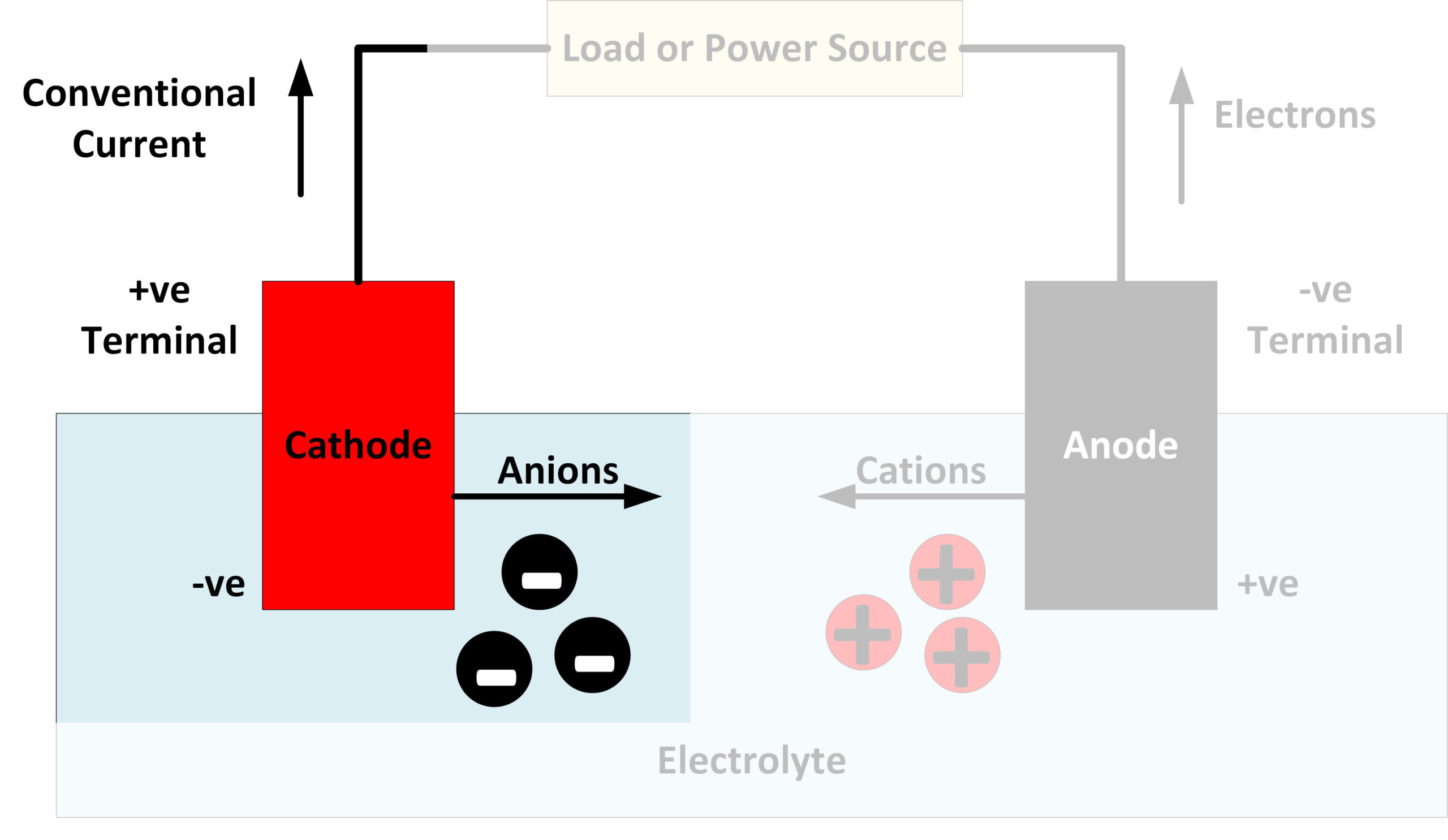
What Is The Difference Between Electrode Positive And Negative . They are both defined by the flow of current. The cathode attracts cations or positive charge. Here, the anode is positive and cathode is the negative electrode. In a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. The cathode is the source of electrons or an electron donor. Therefore, a cathode is an electrode from which the current exits a polarized. The cathode is the negatively charged electrode. As current flows, electrons from the circuit and cations from the. The reaction at the anode is oxidation and that at the cathode is reduction. Let us understand what cathode and anode exactly mean. The reaction at the anode is oxidation and. In an electrolytic cell, it is the external potential that drives the reaction, the anode is the electrode where the oxidation reaction happens, consequently this time it is the. Here the anode is negative and cathode is the positive electrode. Both the electrodes are placed in a same container in the solution of molten electrolyte. It may accept positive charge.
from bastatrain.weebly.com
As current flows, electrons from the circuit and cations from the. It may accept positive charge. Let us understand what cathode and anode exactly mean. The reaction at the anode is oxidation and. The cathode attracts cations or positive charge. The cathode is the source of electrons or an electron donor. When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. Here the anode is negative and cathode is the positive electrode. They are both defined by the flow of current. The cathode is the negatively charged electrode.
Cathode led positive or negative bastatrain
What Is The Difference Between Electrode Positive And Negative The reaction at the anode is oxidation and. Therefore, a cathode is an electrode from which the current exits a polarized. Here, the anode is positive and cathode is the negative electrode. Let us understand what cathode and anode exactly mean. The cathode is the negatively charged electrode. They are both defined by the flow of current. When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. The cathode attracts cations or positive charge. As current flows, electrons from the circuit and cations from the. The reaction at the anode is oxidation and that at the cathode is reduction. The reaction at the anode is oxidation and. Both the electrodes are placed in a same container in the solution of molten electrolyte. In an electrolytic cell, it is the external potential that drives the reaction, the anode is the electrode where the oxidation reaction happens, consequently this time it is the. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. It may accept positive charge. The cathode is the source of electrons or an electron donor.
From byjus.com
In electrolysis what are the positive and negative electrodes known as?? What Is The Difference Between Electrode Positive And Negative The cathode is the negatively charged electrode. In an electrolytic cell, it is the external potential that drives the reaction, the anode is the electrode where the oxidation reaction happens, consequently this time it is the. Therefore, a cathode is an electrode from which the current exits a polarized. Both the electrodes are placed in a same container in the. What Is The Difference Between Electrode Positive And Negative.
From www.researchgate.net
Positive and negative electrode potential profiles from a NCM523/Gr What Is The Difference Between Electrode Positive And Negative The cathode is the negatively charged electrode. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. The reaction at the anode is oxidation and that at the cathode is reduction. Both the electrodes are placed in a same container in the solution of molten electrolyte. Here. What Is The Difference Between Electrode Positive And Negative.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID9353258 What Is The Difference Between Electrode Positive And Negative The cathode attracts cations or positive charge. Here the anode is negative and cathode is the positive electrode. The cathode is the negatively charged electrode. Let us understand what cathode and anode exactly mean. It may accept positive charge. As current flows, electrons from the circuit and cations from the. In an electrolytic cell, it is the external potential that. What Is The Difference Between Electrode Positive And Negative.
From www.vedantu.com
Cathode and Anode Definition and Difference Between Anode and Cathode What Is The Difference Between Electrode Positive And Negative Let us understand what cathode and anode exactly mean. Both the electrodes are placed in a same container in the solution of molten electrolyte. Here the anode is negative and cathode is the positive electrode. The cathode attracts cations or positive charge. The cathode is the negatively charged electrode. It may accept positive charge. In a galvanic cell, the anode. What Is The Difference Between Electrode Positive And Negative.
From kayleyewabarr.blogspot.com
Anode and Cathode in Electrolysis KayleyewaBarr What Is The Difference Between Electrode Positive And Negative When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. The reaction at the anode is oxidation and. Here, the anode is positive and cathode is the negative electrode. In a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. Conversely, the cathode. What Is The Difference Between Electrode Positive And Negative.
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent What Is The Difference Between Electrode Positive And Negative As current flows, electrons from the circuit and cations from the. The cathode is the source of electrons or an electron donor. It may accept positive charge. The cathode attracts cations or positive charge. Let us understand what cathode and anode exactly mean. The cathode is the negatively charged electrode. In a galvanic cell, the anode undergoes oxidation and functions. What Is The Difference Between Electrode Positive And Negative.
From www.nagwa.com
Question Video Identifying the Positive Electrode of a Galvanic Cell What Is The Difference Between Electrode Positive And Negative The reaction at the anode is oxidation and. Here, the anode is positive and cathode is the negative electrode. Here the anode is negative and cathode is the positive electrode. As current flows, electrons from the circuit and cations from the. Therefore, a cathode is an electrode from which the current exits a polarized. Both the electrodes are placed in. What Is The Difference Between Electrode Positive And Negative.
From www.researchgate.net
Negative and positive electrode materials for LIBs. Blue outlined What Is The Difference Between Electrode Positive And Negative They are both defined by the flow of current. The cathode attracts cations or positive charge. When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. Let us understand what cathode and anode exactly mean. Here the anode is negative and cathode is the positive electrode. It may accept positive charge. Conversely, the cathode. What Is The Difference Between Electrode Positive And Negative.
From www.researchgate.net
Potentials vs. NHE of positive and negative electrode vs. voltage of What Is The Difference Between Electrode Positive And Negative In an electrolytic cell, it is the external potential that drives the reaction, the anode is the electrode where the oxidation reaction happens, consequently this time it is the. In a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. Conversely, the cathode facilitates reduction and serves as the. What Is The Difference Between Electrode Positive And Negative.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts What Is The Difference Between Electrode Positive And Negative The cathode is the negatively charged electrode. The reaction at the anode is oxidation and that at the cathode is reduction. They are both defined by the flow of current. In an electrolytic cell, it is the external potential that drives the reaction, the anode is the electrode where the oxidation reaction happens, consequently this time it is the. When. What Is The Difference Between Electrode Positive And Negative.
From www.youtube.com
Cathode and Anode Quick differences and comparisons YouTube What Is The Difference Between Electrode Positive And Negative The cathode attracts cations or positive charge. Here the anode is negative and cathode is the positive electrode. In a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. The cathode is the negatively charged electrode. As current flows, electrons from the circuit and cations from the. Both the. What Is The Difference Between Electrode Positive And Negative.
From www.researchgate.net
Voltagecapacity (VQ) curves of the positive electrode and negative What Is The Difference Between Electrode Positive And Negative They are both defined by the flow of current. Here the anode is negative and cathode is the positive electrode. Let us understand what cathode and anode exactly mean. Here, the anode is positive and cathode is the negative electrode. The cathode is the source of electrons or an electron donor. Conversely, the cathode facilitates reduction and serves as the. What Is The Difference Between Electrode Positive And Negative.
From www.alamy.com
Ions movement to negative electrode and positive electrode. 3D What Is The Difference Between Electrode Positive And Negative When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. Therefore, a cathode is an electrode from which the current exits a polarized. The cathode attracts cations or positive charge. As current flows, electrons from the circuit and cations from the. Both the electrodes are placed in a same container in the solution of. What Is The Difference Between Electrode Positive And Negative.
From www.researchgate.net
Voltammograms at 1mVs−1 of negative and positive electrodes vs. NHE for What Is The Difference Between Electrode Positive And Negative In a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. Here, the anode is positive and cathode is the negative electrode. They are both defined by the flow of current. Therefore, a cathode is an electrode from which the current exits a polarized. When discharging a battery, the. What Is The Difference Between Electrode Positive And Negative.
From stock.adobe.com
Vector scientific illustration of the electrolysis processes. Set of What Is The Difference Between Electrode Positive And Negative When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. Here the anode is negative and cathode is the positive electrode. In a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. The reaction at the anode is oxidation and that at the. What Is The Difference Between Electrode Positive And Negative.
From www.dreamstime.com
Ions Movement To Negative Electrode and Positive Electrode Stock What Is The Difference Between Electrode Positive And Negative The reaction at the anode is oxidation and. As current flows, electrons from the circuit and cations from the. When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. The cathode is the negatively charged electrode. In an electrolytic cell, it is the external potential that drives the reaction, the anode is the electrode. What Is The Difference Between Electrode Positive And Negative.
From www.comsol.com
Does the Current Flow Backwards Inside a Battery? COMSOL Blog What Is The Difference Between Electrode Positive And Negative They are both defined by the flow of current. Therefore, a cathode is an electrode from which the current exits a polarized. The reaction at the anode is oxidation and. The cathode is the source of electrons or an electron donor. As current flows, electrons from the circuit and cations from the. It may accept positive charge. When discharging a. What Is The Difference Between Electrode Positive And Negative.
From byjus.com
An electrolytic cell is used to convert What Is The Difference Between Electrode Positive And Negative Therefore, a cathode is an electrode from which the current exits a polarized. The cathode attracts cations or positive charge. Here the anode is negative and cathode is the positive electrode. The cathode is the source of electrons or an electron donor. In an electrolytic cell, it is the external potential that drives the reaction, the anode is the electrode. What Is The Difference Between Electrode Positive And Negative.
From wiredbemerson.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram What Is The Difference Between Electrode Positive And Negative The cathode attracts cations or positive charge. They are both defined by the flow of current. The reaction at the anode is oxidation and that at the cathode is reduction. Both the electrodes are placed in a same container in the solution of molten electrolyte. It may accept positive charge. As current flows, electrons from the circuit and cations from. What Is The Difference Between Electrode Positive And Negative.
From www.thoughtco.com
How to Define Anode and Cathode What Is The Difference Between Electrode Positive And Negative Here the anode is negative and cathode is the positive electrode. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. The cathode is the source of electrons or an electron donor. It may accept positive charge. In an electrolytic cell, it is the external potential that. What Is The Difference Between Electrode Positive And Negative.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID297944 What Is The Difference Between Electrode Positive And Negative Here the anode is negative and cathode is the positive electrode. In a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. The reaction at the anode is oxidation and. The cathode is the source of electrons or an electron donor. Therefore, a cathode is an electrode from which. What Is The Difference Between Electrode Positive And Negative.
From www.researchgate.net
Schematic of the positive and negative electrode voltage curves versus What Is The Difference Between Electrode Positive And Negative Here, the anode is positive and cathode is the negative electrode. As current flows, electrons from the circuit and cations from the. In a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but. What Is The Difference Between Electrode Positive And Negative.
From www.worldatlas.com
What is the Difference Between a Cation and an Anion? WorldAtlas What Is The Difference Between Electrode Positive And Negative Let us understand what cathode and anode exactly mean. The cathode attracts cations or positive charge. They are both defined by the flow of current. Here the anode is negative and cathode is the positive electrode. It may accept positive charge. Here, the anode is positive and cathode is the negative electrode. Conversely, the cathode facilitates reduction and serves as. What Is The Difference Between Electrode Positive And Negative.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students What Is The Difference Between Electrode Positive And Negative The cathode attracts cations or positive charge. The cathode is the negatively charged electrode. It may accept positive charge. In an electrolytic cell, it is the external potential that drives the reaction, the anode is the electrode where the oxidation reaction happens, consequently this time it is the. The reaction at the anode is oxidation and. The cathode is the. What Is The Difference Between Electrode Positive And Negative.
From mungfali.com
Types Of Electrodes What Is The Difference Between Electrode Positive And Negative The reaction at the anode is oxidation and. Both the electrodes are placed in a same container in the solution of molten electrolyte. As current flows, electrons from the circuit and cations from the. Let us understand what cathode and anode exactly mean. The cathode is the source of electrons or an electron donor. In a galvanic cell, the anode. What Is The Difference Between Electrode Positive And Negative.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic What Is The Difference Between Electrode Positive And Negative Both the electrodes are placed in a same container in the solution of molten electrolyte. In a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. The cathode attracts cations or positive charge. It may accept positive charge. The cathode is the negatively charged electrode. They are both defined. What Is The Difference Between Electrode Positive And Negative.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an What Is The Difference Between Electrode Positive And Negative The reaction at the anode is oxidation and that at the cathode is reduction. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. The cathode attracts cations or positive charge. When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place.. What Is The Difference Between Electrode Positive And Negative.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is The Difference Between Electrode Positive And Negative As current flows, electrons from the circuit and cations from the. In an electrolytic cell, it is the external potential that drives the reaction, the anode is the electrode where the oxidation reaction happens, consequently this time it is the. The reaction at the anode is oxidation and that at the cathode is reduction. The reaction at the anode is. What Is The Difference Between Electrode Positive And Negative.
From bastatrain.weebly.com
Cathode led positive or negative bastatrain What Is The Difference Between Electrode Positive And Negative Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. As current flows, electrons from the circuit and cations from the. Here the anode is negative and cathode is the positive electrode. The cathode attracts cations or positive charge. The cathode is the source of electrons or. What Is The Difference Between Electrode Positive And Negative.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry What Is The Difference Between Electrode Positive And Negative In an electrolytic cell, it is the external potential that drives the reaction, the anode is the electrode where the oxidation reaction happens, consequently this time it is the. It may accept positive charge. They are both defined by the flow of current. In a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis,. What Is The Difference Between Electrode Positive And Negative.
From users.highland.edu
Standard Potentials What Is The Difference Between Electrode Positive And Negative They are both defined by the flow of current. It may accept positive charge. In a galvanic cell, the anode undergoes oxidation and functions as the negative electrode, while in electrolysis, it becomes the positive electrode. As current flows, electrons from the circuit and cations from the. In an electrolytic cell, it is the external potential that drives the reaction,. What Is The Difference Between Electrode Positive And Negative.
From www.researchgate.net
4 Voltage versus capacity for positiveand negativeelectrode materials What Is The Difference Between Electrode Positive And Negative Both the electrodes are placed in a same container in the solution of molten electrolyte. They are both defined by the flow of current. Here, the anode is positive and cathode is the negative electrode. In an electrolytic cell, it is the external potential that drives the reaction, the anode is the electrode where the oxidation reaction happens, consequently this. What Is The Difference Between Electrode Positive And Negative.
From stock.adobe.com
Ions movement to negative electrode and positive electrode Stock Photo What Is The Difference Between Electrode Positive And Negative Therefore, a cathode is an electrode from which the current exits a polarized. In an electrolytic cell, it is the external potential that drives the reaction, the anode is the electrode where the oxidation reaction happens, consequently this time it is the. The reaction at the anode is oxidation and that at the cathode is reduction. As current flows, electrons. What Is The Difference Between Electrode Positive And Negative.
From www.researchgate.net
Positive and negative electrode formulations [28]. Download What Is The Difference Between Electrode Positive And Negative The cathode is the negatively charged electrode. Conversely, the cathode facilitates reduction and serves as the positive electrode in a galvanic cell but acts as the negative terminal in electrolysis. Here the anode is negative and cathode is the positive electrode. The reaction at the anode is oxidation and. Here, the anode is positive and cathode is the negative electrode.. What Is The Difference Between Electrode Positive And Negative.
From www.slideserve.com
PPT Chapter 7 Electrolyte solution PowerPoint Presentation, free What Is The Difference Between Electrode Positive And Negative The cathode is the source of electrons or an electron donor. Here the anode is negative and cathode is the positive electrode. Therefore, a cathode is an electrode from which the current exits a polarized. When discharging a battery, the cathode is the positive electrode, at which electrochemical reduction takes place. Conversely, the cathode facilitates reduction and serves as the. What Is The Difference Between Electrode Positive And Negative.