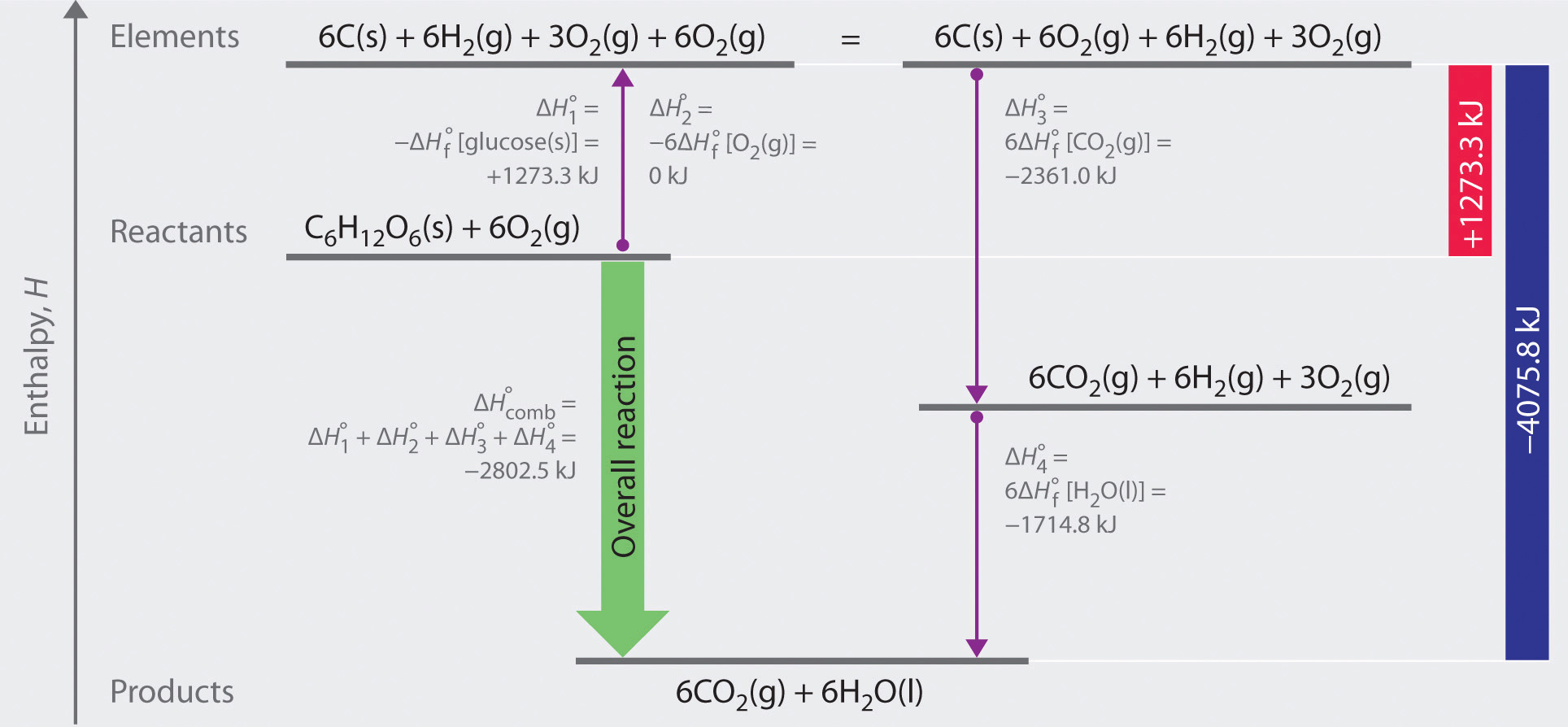
Define Standard Enthalpy Of Formation . All chemical reactions involve a change in enthalpy (defined as the heat produced or. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Find out the difference between enthalpy of formation,. The symbol for the standard enthalpy of formation is: Learn how to calculate and interpret the standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from. Learn what standard enthalpy of formation is and how to calculate it for different reactions. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen.
from chem.libretexts.org
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. The symbol for the standard enthalpy of formation is: Learn how to calculate and interpret the standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from. Find out the difference between enthalpy of formation,. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. All chemical reactions involve a change in enthalpy (defined as the heat produced or. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Learn what standard enthalpy of formation is and how to calculate it for different reactions.
5.7 Enthalpies of Formation Chemistry LibreTexts
Define Standard Enthalpy Of Formation The symbol for the standard enthalpy of formation is: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The symbol for the standard enthalpy of formation is: Find out the difference between enthalpy of formation,. Learn how to calculate and interpret the standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. All chemical reactions involve a change in enthalpy (defined as the heat produced or. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. Learn what standard enthalpy of formation is and how to calculate it for different reactions.
From www.slideserve.com
PPT Energy, Enthalpy, and Thermochemistry PowerPoint Presentation Define Standard Enthalpy Of Formation Learn how to calculate and interpret the standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The symbol for the standard enthalpy of formation is: Learn. Define Standard Enthalpy Of Formation.
From studylib.net
Standard Enthalpy of Formation and Reaction Define Standard Enthalpy Of Formation Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. All chemical reactions involve a change in enthalpy (defined as the heat produced or. Learn what standard enthalpy of formation is and how to calculate it for different. Define Standard Enthalpy Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Define Standard Enthalpy Of Formation Learn what standard enthalpy of formation is and how to calculate it for different reactions. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. The. Define Standard Enthalpy Of Formation.
From rayb78.github.io
Heat Of Formation Chart Define Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Learn what standard enthalpy of formation is and how to calculate it for different reactions. Learn how to calculate and interpret the standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of. Define Standard Enthalpy Of Formation.
From www.slideshare.net
Standard enthalpy of formation Define Standard Enthalpy Of Formation Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. All chemical reactions involve a change in enthalpy (defined as the heat produced or. Find out the difference between enthalpy of formation,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Define Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Define Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The symbol for the standard enthalpy of formation is: Learn what standard enthalpy of formation is and how to calculate it for different reactions. All chemical reactions involve a change in enthalpy (defined as the heat produced. Define Standard Enthalpy Of Formation.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Define Standard Enthalpy Of Formation Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. Learn what standard enthalpy of formation is and how to calculate it for different reactions. The symbol for the standard enthalpy of formation is: All chemical reactions involve a change in enthalpy (defined as the heat produced or. 193. Define Standard Enthalpy Of Formation.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Define Standard Enthalpy Of Formation Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. Learn how to calculate and interpret the standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from. All chemical reactions involve a change in enthalpy (defined as the heat produced or.. Define Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Define Standard Enthalpy Of Formation Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is defined as the change in enthalpy when one mole of. Define Standard Enthalpy Of Formation.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Define Standard Enthalpy Of Formation The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Learn how to. Define Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free Define Standard Enthalpy Of Formation All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Learn what standard enthalpy of formation is and how to calculate it for different reactions. Find out the difference between enthalpy. Define Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Enthalpy (H) PowerPoint Presentation, free download ID305168 Define Standard Enthalpy Of Formation The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Learn what standard enthalpy of formation is and how to calculate it for different reactions. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is. Define Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Define Standard Enthalpy Of Formation The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The symbol for the standard enthalpy of formation is: Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. Find out the difference between. Define Standard Enthalpy Of Formation.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Define Standard Enthalpy Of Formation Learn what standard enthalpy of formation is and how to calculate it for different reactions. All chemical reactions involve a change in enthalpy (defined as the heat produced or. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation, also known as heat of. Define Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Define Standard Enthalpy Of Formation The symbol for the standard enthalpy of formation is: The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. Learn what standard enthalpy of. Define Standard Enthalpy Of Formation.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Define Standard Enthalpy Of Formation The symbol for the standard enthalpy of formation is: Learn what standard enthalpy of formation is and how to calculate it for different reactions. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. The standard enthalpy of formation is defined as the change in enthalpy when one mole. Define Standard Enthalpy Of Formation.
From quizlet.com
Given the definition of the standard enthalpy of formation f Quizlet Define Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. The standard enthalpy of formation is defined as the change in enthalpy when one mole of. Define Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Enthalpies of Formation and Reaction PowerPoint Presentation Define Standard Enthalpy Of Formation The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Learn how to. Define Standard Enthalpy Of Formation.
From slidetodoc.com
ENTHALPY CHANGES ENTHALPY A guide for A level Define Standard Enthalpy Of Formation Learn what standard enthalpy of formation is and how to calculate it for different reactions. Learn how to calculate and interpret the standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is. Define Standard Enthalpy Of Formation.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Define Standard Enthalpy Of Formation Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The symbol for the standard enthalpy of formation is: Learn how to calculate and. Define Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Energy changes PowerPoint Presentation, free download ID3003657 Define Standard Enthalpy Of Formation Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The symbol for the standard enthalpy of. Define Standard Enthalpy Of Formation.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Define Standard Enthalpy Of Formation The symbol for the standard enthalpy of formation is: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. All chemical reactions. Define Standard Enthalpy Of Formation.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Define Standard Enthalpy Of Formation All chemical reactions involve a change in enthalpy (defined as the heat produced or. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Define Standard Enthalpy Of Formation.
From www.youtube.com
5.1 Define Standard State, Enthalpy change of formation and combustion Define Standard Enthalpy Of Formation Learn how to calculate and interpret the standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. The standard enthalpy of formation is defined as the change in enthalpy when one. Define Standard Enthalpy Of Formation.
From radenbayu33as.blogspot.com
Enthalpy Of Formation Of C3H8 Enthalpy Changes during Formation of Define Standard Enthalpy Of Formation All chemical reactions involve a change in enthalpy (defined as the heat produced or. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Find out the difference between enthalpy of formation,. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures. Define Standard Enthalpy Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Define Standard Enthalpy Of Formation Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Learn how to calculate and interpret the standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from. The standard enthalpy. Define Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID3528115 Define Standard Enthalpy Of Formation Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. The standard enthalpy of formation is defined. Define Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Define Standard Enthalpy Of Formation Learn how to calculate and interpret the standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from. Find out the difference between enthalpy of formation,. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. All chemical reactions involve a change. Define Standard Enthalpy Of Formation.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Define Standard Enthalpy Of Formation Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. Find out the difference between enthalpy of. Define Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Define Standard Enthalpy Of Formation All chemical reactions involve a change in enthalpy (defined as the heat produced or. Learn how to calculate and interpret the standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one.. Define Standard Enthalpy Of Formation.
From studymarxianism.z21.web.core.windows.net
How To Find Standard Enthalpy Define Standard Enthalpy Of Formation Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. All chemical reactions involve a change in enthalpy (defined as the heat produced or. Learn how to calculate and interpret the standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from.. Define Standard Enthalpy Of Formation.
From www.nagwa.com
Question Video Determining the Standard Enthalpy of Formation of a Define Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Learn how to calculate and interpret the standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from. Enthalpy of formation (δhf) is the enthalpy change for the formation of. Define Standard Enthalpy Of Formation.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Define Standard Enthalpy Of Formation Learn how to calculate and interpret the standard enthalpy of formation, an enthalpy change for a reaction that forms 1 mole of a substance from. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one. Learn what standard enthalpy of formation is and how to calculate it for different. Define Standard Enthalpy Of Formation.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Define Standard Enthalpy Of Formation The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. All chemical reactions involve a change in enthalpy (defined as the heat produced or. Enthalpy of formation, also known as heat of formation, is a thermodynamic property that measures the heat energy change when one.. Define Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Define Standard Enthalpy Of Formation Learn what standard enthalpy of formation is and how to calculate it for different reactions. Find out the difference between enthalpy of formation,. The symbol for the standard enthalpy of formation is: The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. All chemical reactions. Define Standard Enthalpy Of Formation.