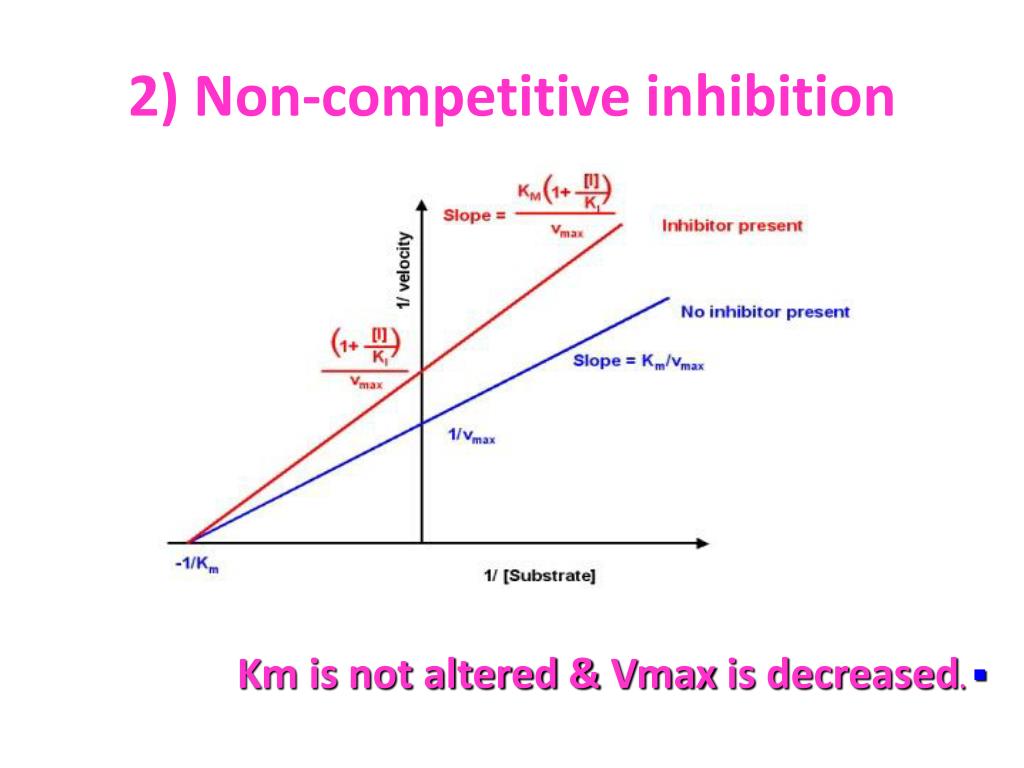
Competitive Inhibition Effect On Vmax . In effect, they compete for the active site and bind in a mutually exclusive fashion. The effect on kinetics is as if the enzyme were less active (vmax is reduced), but that the affinity for substrate is unaffected (km remains the same) since the substrate binding site is not occupied by the noncompetitive inhibitor. most undergraduate biochemistry textbooks note that uncompetitive inhibitors lower both vmax and km by the same factor, α′ 1. This is illustrated in the chemical equations and molecular cartoons shown in figure 6.4.1. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for. At [i] = ki , km, apparent = 2 x km. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for the enzyme to. inhibition cannot be overcome by increasing the concentration of s. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. note the effect of 1+[i]/ki on km: As [i] increases, km, apparent = km (1 + [i]/ki) increases; thus, a competitive inhibitor does not affect the maximum activity (vmax) of. K 3 forward and k. the competitive inhibition model is an extension, where inhibitor can bind reversibly to enzyme (i.e.
from www.slideserve.com
thus, a competitive inhibitor does not affect the maximum activity (vmax) of. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for the enzyme to. In effect, they compete for the active site and bind in a mutually exclusive fashion. This is illustrated in the chemical equations and molecular cartoons shown in figure 6.4.1. At [i] = ki , km, apparent = 2 x km. The effect on kinetics is as if the enzyme were less active (vmax is reduced), but that the affinity for substrate is unaffected (km remains the same) since the substrate binding site is not occupied by the noncompetitive inhibitor. note the effect of 1+[i]/ki on km: K 3 forward and k. As [i] increases, km, apparent = km (1 + [i]/ki) increases; reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme.
PPT Inhibition of enzyme activity PowerPoint Presentation, free
Competitive Inhibition Effect On Vmax most undergraduate biochemistry textbooks note that uncompetitive inhibitors lower both vmax and km by the same factor, α′ 1. most undergraduate biochemistry textbooks note that uncompetitive inhibitors lower both vmax and km by the same factor, α′ 1. The effect on kinetics is as if the enzyme were less active (vmax is reduced), but that the affinity for substrate is unaffected (km remains the same) since the substrate binding site is not occupied by the noncompetitive inhibitor. note the effect of 1+[i]/ki on km: At [i] = ki , km, apparent = 2 x km. thus, a competitive inhibitor does not affect the maximum activity (vmax) of. As [i] increases, km, apparent = km (1 + [i]/ki) increases; inhibition cannot be overcome by increasing the concentration of s. K 3 forward and k. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for the enzyme to. the competitive inhibition model is an extension, where inhibitor can bind reversibly to enzyme (i.e. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. This is illustrated in the chemical equations and molecular cartoons shown in figure 6.4.1. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for. In effect, they compete for the active site and bind in a mutually exclusive fashion.
From davlqrbxeco.blob.core.windows.net
Competitive Inhibition Vmax Remains at David Jenkins blog Competitive Inhibition Effect On Vmax In effect, they compete for the active site and bind in a mutually exclusive fashion. thus, a competitive inhibitor does not affect the maximum activity (vmax) of. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. As [i] increases, km, apparent = km (1 + [i]/ki) increases; The. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT Lecture Notes for Chapter 7 Enzyme and Inhibition Competitive Inhibition Effect On Vmax reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. The effect on kinetics is as if the enzyme were less active (vmax is reduced), but that the affinity for substrate is unaffected (km remains the same) since the substrate binding site is not occupied by the noncompetitive inhibitor. . Competitive Inhibition Effect On Vmax.
From faaiznarendra.blogspot.com
47+ how to calculate ki for competitive inhibition FaaizNarendra Competitive Inhibition Effect On Vmax This is illustrated in the chemical equations and molecular cartoons shown in figure 6.4.1. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. K 3 forward and k. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for. inhibition cannot. Competitive Inhibition Effect On Vmax.
From www.animalia-life.club
Mixed Inhibition Graph Competitive Inhibition Effect On Vmax At [i] = ki , km, apparent = 2 x km. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for the enzyme to. In effect, they compete for the active site and bind in a mutually exclusive fashion. notice that at high substrate concentrations, the competitive inhibitor has essentially no. Competitive Inhibition Effect On Vmax.
From slideplayer.com
Packet 13 Campbell—Chapter 8 ppt download Competitive Inhibition Effect On Vmax notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for the enzyme to. As [i] increases, km, apparent = km (1 + [i]/ki) increases; In effect, they compete for the active site and bind in a mutually exclusive fashion. K 3 forward and k. most undergraduate biochemistry textbooks note that uncompetitive. Competitive Inhibition Effect On Vmax.
From www.lecturio.com
Enzyme Inhibition Concise Medical Knowledge Competitive Inhibition Effect On Vmax In effect, they compete for the active site and bind in a mutually exclusive fashion. This is illustrated in the chemical equations and molecular cartoons shown in figure 6.4.1. the competitive inhibition model is an extension, where inhibitor can bind reversibly to enzyme (i.e. thus, a competitive inhibitor does not affect the maximum activity (vmax) of. notice. Competitive Inhibition Effect On Vmax.
From davlqrbxeco.blob.core.windows.net
Competitive Inhibition Vmax Remains at David Jenkins blog Competitive Inhibition Effect On Vmax At [i] = ki , km, apparent = 2 x km. the competitive inhibition model is an extension, where inhibitor can bind reversibly to enzyme (i.e. inhibition cannot be overcome by increasing the concentration of s. As [i] increases, km, apparent = km (1 + [i]/ki) increases; thus, a competitive inhibitor does not affect the maximum activity. Competitive Inhibition Effect On Vmax.
From www.sigmaaldrich.com
Reversible Inhibitors Competitive Inhibition Effect On Vmax notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for the enzyme to. The effect on kinetics is as if the enzyme were less active (vmax is reduced), but that the affinity for substrate is unaffected (km remains the same) since the substrate binding site is not occupied by the noncompetitive inhibitor.. Competitive Inhibition Effect On Vmax.
From www.researchgate.net
Competitive inhibition. In the direct plot of initial velocity (v i Competitive Inhibition Effect On Vmax The effect on kinetics is as if the enzyme were less active (vmax is reduced), but that the affinity for substrate is unaffected (km remains the same) since the substrate binding site is not occupied by the noncompetitive inhibitor. note the effect of 1+[i]/ki on km: As [i] increases, km, apparent = km (1 + [i]/ki) increases; notice. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT Lecture Notes for Chapter 7 Enzyme and Inhibition Competitive Inhibition Effect On Vmax In effect, they compete for the active site and bind in a mutually exclusive fashion. inhibition cannot be overcome by increasing the concentration of s. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for the enzyme to. The effect on kinetics is as if the enzyme were less active (vmax. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT Lecture 7Enzyme InhibitionDrug Discovery PowerPoint Competitive Inhibition Effect On Vmax notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for the enzyme to. the competitive inhibition model is an extension, where inhibitor can bind reversibly to enzyme (i.e. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. At [i] =. Competitive Inhibition Effect On Vmax.
From slideplayer.com
Packet 13 Campbell—Chapter 8 ppt download Competitive Inhibition Effect On Vmax most undergraduate biochemistry textbooks note that uncompetitive inhibitors lower both vmax and km by the same factor, α′ 1. This is illustrated in the chemical equations and molecular cartoons shown in figure 6.4.1. In effect, they compete for the active site and bind in a mutually exclusive fashion. thus, a competitive inhibitor does not affect the maximum activity. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT Inhibition of enzyme activity PowerPoint Presentation, free Competitive Inhibition Effect On Vmax the competitive inhibition model is an extension, where inhibitor can bind reversibly to enzyme (i.e. note the effect of 1+[i]/ki on km: most undergraduate biochemistry textbooks note that uncompetitive inhibitors lower both vmax and km by the same factor, α′ 1. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT Lecture 16 PowerPoint Presentation, free download ID559678 Competitive Inhibition Effect On Vmax most undergraduate biochemistry textbooks note that uncompetitive inhibitors lower both vmax and km by the same factor, α′ 1. The effect on kinetics is as if the enzyme were less active (vmax is reduced), but that the affinity for substrate is unaffected (km remains the same) since the substrate binding site is not occupied by the noncompetitive inhibitor. This. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT Enzymes Basic Concepts and PowerPoint Presentation Competitive Inhibition Effect On Vmax As [i] increases, km, apparent = km (1 + [i]/ki) increases; notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for the enzyme to. This is illustrated in the chemical equations and molecular cartoons shown in figure 6.4.1. The effect on kinetics is as if the enzyme were less active (vmax is. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT Enzyme Inhibition PowerPoint Presentation ID1079722 Competitive Inhibition Effect On Vmax notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for the enzyme to. note the effect of 1+[i]/ki on km: As [i] increases, km, apparent = km (1 + [i]/ki) increases; the competitive inhibition model is an extension, where inhibitor can bind reversibly to enzyme (i.e. thus, a competitive. Competitive Inhibition Effect On Vmax.
From www.youtube.com
Inhibition YouTube Competitive Inhibition Effect On Vmax thus, a competitive inhibitor does not affect the maximum activity (vmax) of. inhibition cannot be overcome by increasing the concentration of s. the competitive inhibition model is an extension, where inhibitor can bind reversibly to enzyme (i.e. most undergraduate biochemistry textbooks note that uncompetitive inhibitors lower both vmax and km by the same factor, α′ 1.. Competitive Inhibition Effect On Vmax.
From www.chegg.com
Solved 2) how does the value of V max for the enzyme compare Competitive Inhibition Effect On Vmax thus, a competitive inhibitor does not affect the maximum activity (vmax) of. This is illustrated in the chemical equations and molecular cartoons shown in figure 6.4.1. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the. Competitive Inhibition Effect On Vmax.
From slidetodoc.com
II PROTEIN BIOCHEMISTRY 2 6 Enzyme 2 Competitive Inhibition Effect On Vmax As [i] increases, km, apparent = km (1 + [i]/ki) increases; The effect on kinetics is as if the enzyme were less active (vmax is reduced), but that the affinity for substrate is unaffected (km remains the same) since the substrate binding site is not occupied by the noncompetitive inhibitor. At [i] = ki , km, apparent = 2 x. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT ENZYMES INHIBITION, REGULATION PowerPoint Presentation Competitive Inhibition Effect On Vmax thus, a competitive inhibitor does not affect the maximum activity (vmax) of. note the effect of 1+[i]/ki on km: In effect, they compete for the active site and bind in a mutually exclusive fashion. As [i] increases, km, apparent = km (1 + [i]/ki) increases; notice that at high substrate concentrations, the competitive inhibitor has essentially no. Competitive Inhibition Effect On Vmax.
From schoolbag.info
Biochemistry MCAT Biology and Biochemistry Competitive Inhibition Effect On Vmax This is illustrated in the chemical equations and molecular cartoons shown in figure 6.4.1. At [i] = ki , km, apparent = 2 x km. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for. As [i] increases, km, apparent = km (1 + [i]/ki) increases; inhibition cannot be overcome by. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT ENZYMES INHIBITION, REGULATION PowerPoint Presentation Competitive Inhibition Effect On Vmax notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for the enzyme to. In effect, they compete for the active site and bind in a mutually exclusive fashion. The effect on kinetics is as if the enzyme were less active (vmax is reduced), but that the affinity for substrate is unaffected (km. Competitive Inhibition Effect On Vmax.
From www.gkseries.com
In competitive inhibition Vmax Competitive Inhibition Effect On Vmax most undergraduate biochemistry textbooks note that uncompetitive inhibitors lower both vmax and km by the same factor, α′ 1. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for. thus, a competitive inhibitor does not affect the maximum activity (vmax) of. notice that at high substrate concentrations, the competitive. Competitive Inhibition Effect On Vmax.
From slideplayer.com
Lecture 8 Enzyme ppt download Competitive Inhibition Effect On Vmax In effect, they compete for the active site and bind in a mutually exclusive fashion. the competitive inhibition model is an extension, where inhibitor can bind reversibly to enzyme (i.e. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for the enzyme to. notice that at high substrate concentrations, the. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT Inhibition of enzyme activity PowerPoint Presentation, free Competitive Inhibition Effect On Vmax This is illustrated in the chemical equations and molecular cartoons shown in figure 6.4.1. note the effect of 1+[i]/ki on km: reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. thus, a competitive inhibitor does not affect the maximum activity (vmax) of. The effect on kinetics is. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT HOW ENZYMES WORK PowerPoint Presentation, free download ID6954410 Competitive Inhibition Effect On Vmax inhibition cannot be overcome by increasing the concentration of s. most undergraduate biochemistry textbooks note that uncompetitive inhibitors lower both vmax and km by the same factor, α′ 1. K 3 forward and k. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for. The effect on kinetics is as. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT Enzymes Basic Concepts and PowerPoint Presentation Competitive Inhibition Effect On Vmax This is illustrated in the chemical equations and molecular cartoons shown in figure 6.4.1. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. K 3 forward and k. note the effect of 1+[i]/ki on km: As [i] increases, km, apparent = km (1 + [i]/ki) increases; At [i]. Competitive Inhibition Effect On Vmax.
From dxoaogqyv.blob.core.windows.net
Competitive Inhibition And Substrate Concentration at James Williams blog Competitive Inhibition Effect On Vmax This is illustrated in the chemical equations and molecular cartoons shown in figure 6.4.1. inhibition cannot be overcome by increasing the concentration of s. In effect, they compete for the active site and bind in a mutually exclusive fashion. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for. As [i]. Competitive Inhibition Effect On Vmax.
From slideplayer.com
The MichaelisMenton Model ppt download Competitive Inhibition Effect On Vmax notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. K 3 forward and k. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax. Competitive Inhibition Effect On Vmax.
From thechemistrynotes.com
Enzymes Definition, Classification, Action, Inhibition, Functions Competitive Inhibition Effect On Vmax notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for the enzyme to. At [i] = ki , km, apparent = 2 x km. inhibition cannot be overcome by increasing the concentration of s. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT Enzyme Inhibition PowerPoint Presentation, free Competitive Inhibition Effect On Vmax inhibition cannot be overcome by increasing the concentration of s. This is illustrated in the chemical equations and molecular cartoons shown in figure 6.4.1. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax for. notice that at high substrate concentrations, the competitive inhibitor has essentially no effect, causing the vmax. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT LECTURE 4 Reaction Mechanisms and Inhibitors PowerPoint Competitive Inhibition Effect On Vmax The effect on kinetics is as if the enzyme were less active (vmax is reduced), but that the affinity for substrate is unaffected (km remains the same) since the substrate binding site is not occupied by the noncompetitive inhibitor. most undergraduate biochemistry textbooks note that uncompetitive inhibitors lower both vmax and km by the same factor, α′ 1. . Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT HOW ENZYMES WORK PowerPoint Presentation, free download ID6954410 Competitive Inhibition Effect On Vmax K 3 forward and k. In effect, they compete for the active site and bind in a mutually exclusive fashion. the competitive inhibition model is an extension, where inhibitor can bind reversibly to enzyme (i.e. As [i] increases, km, apparent = km (1 + [i]/ki) increases; inhibition cannot be overcome by increasing the concentration of s. notice. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT ENZYME PowerPoint Presentation, free download ID250062 Competitive Inhibition Effect On Vmax thus, a competitive inhibitor does not affect the maximum activity (vmax) of. At [i] = ki , km, apparent = 2 x km. As [i] increases, km, apparent = km (1 + [i]/ki) increases; The effect on kinetics is as if the enzyme were less active (vmax is reduced), but that the affinity for substrate is unaffected (km remains. Competitive Inhibition Effect On Vmax.
From www.slideserve.com
PPT V max and Km of Enzymecontrolled Reactions PowerPoint Competitive Inhibition Effect On Vmax This is illustrated in the chemical equations and molecular cartoons shown in figure 6.4.1. thus, a competitive inhibitor does not affect the maximum activity (vmax) of. most undergraduate biochemistry textbooks note that uncompetitive inhibitors lower both vmax and km by the same factor, α′ 1. notice that at high substrate concentrations, the competitive inhibitor has essentially no. Competitive Inhibition Effect On Vmax.