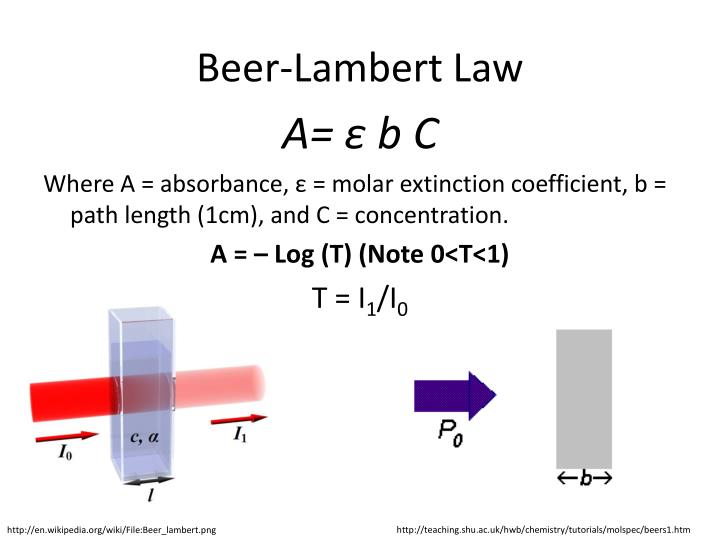
Beer Lambert Law E Value . You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. A higher ε value means stronger absorption at that wavelength. The path length (l) is the physical.
from fyooddpjn.blob.core.windows.net
A higher ε value means stronger absorption at that wavelength. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The path length (l) is the physical.
Beer Lambert Law Enzyme Activity at Russell Bingaman blog
Beer Lambert Law E Value Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. A higher ε value means stronger absorption at that wavelength. The path length (l) is the physical. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer Lambert Law E Value You can also use this calculator to. A higher ε value means stronger absorption at that wavelength. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The path length (l) is the physical. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law E Value.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer Lambert Law E Value The path length (l) is the physical. A higher ε value means stronger absorption at that wavelength. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. In most experiments, molar. Beer Lambert Law E Value.
From www.youtube.com
Derivation of Beer Lambert Law YouTube Beer Lambert Law E Value Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. The path length (l) is the physical. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. A higher ε value means stronger absorption at that wavelength. In most experiments, molar. Beer Lambert Law E Value.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer Lambert Law E Value \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. The path length (l) is the physical. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. A higher ε value means stronger absorption at that wavelength. In most experiments, molar absorptivity (ε) and the length (b) are. Beer Lambert Law E Value.
From www.youtube.com
Worked example Calculating concentration using the BeerLambert law Beer Lambert Law E Value In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The path length (l) is the physical. You can also use this calculator to. A higher ε value means. Beer Lambert Law E Value.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube Beer Lambert Law E Value In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. The path length (l) is the physical. A higher ε value means. Beer Lambert Law E Value.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law E Value \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. A higher ε value means stronger absorption at that wavelength. The path length (l) is the physical. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law E Value.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Beer Lambert Law E Value In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The path length (l) is the physical. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. A higher ε value means. Beer Lambert Law E Value.
From www.youtube.com
Beer and Lambert Law Derivation YouTube Beer Lambert Law E Value \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. A higher ε value means stronger absorption at that wavelength. The path length (l) is the physical. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law E Value.
From www.thoughtco.com
Beer's Law Definition and Equation Beer Lambert Law E Value Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. The path length (l) is the physical. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this. Beer Lambert Law E Value.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer Lambert Law E Value You can also use this calculator to. A higher ε value means stronger absorption at that wavelength. The path length (l) is the physical. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law E Value.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy Beer Lambert Law E Value The path length (l) is the physical. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance. Beer Lambert Law E Value.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer Lambert Law E Value A higher ε value means stronger absorption at that wavelength. The path length (l) is the physical. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law E Value.
From rumble.com
BeerLambert Law, Spectrophotometry, Example Chemistry Beer Lambert Law E Value \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. You can also use this calculator to. The path length (l) is the physical. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. A higher ε value means stronger absorption at that wavelength. In most experiments, molar. Beer Lambert Law E Value.
From www.slideserve.com
PPT Exp 14B Determining an Equilibrium Constant PowerPoint Beer Lambert Law E Value A higher ε value means stronger absorption at that wavelength. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this calculator to. The path length (l) is the physical. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law E Value.
From www.youtube.com
Beer's Law Overview YouTube Beer Lambert Law E Value A higher ε value means stronger absorption at that wavelength. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The path. Beer Lambert Law E Value.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer Lambert Law E Value A higher ε value means stronger absorption at that wavelength. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can. Beer Lambert Law E Value.
From www.youtube.com
Spectrophotometric terms and BeerLambert Law YouTube Beer Lambert Law E Value A higher ε value means stronger absorption at that wavelength. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. In most experiments, molar absorptivity (ε) and the length (b) are. Beer Lambert Law E Value.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Beer Lambert Law E Value Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. A higher ε value means stronger absorption at that wavelength. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. You can. Beer Lambert Law E Value.
From fyooddpjn.blob.core.windows.net
Beer Lambert Law Enzyme Activity at Russell Bingaman blog Beer Lambert Law E Value A higher ε value means stronger absorption at that wavelength. You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. The path length (l) is the physical. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law E Value.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer Lambert Law E Value The path length (l) is the physical. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l. Beer Lambert Law E Value.
From www.youtube.com
B.7 The Beer Lambert law (HL) YouTube Beer Lambert Law E Value In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. A higher ε value means stronger absorption at that wavelength. You can also use this calculator to. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer Lambert Law E Value.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer Lambert Law E Value The path length (l) is the physical. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l. Beer Lambert Law E Value.
From mckennaroscervantes.blogspot.com
Beer's Lambert Law Equation MckennarosCervantes Beer Lambert Law E Value A higher ε value means stronger absorption at that wavelength. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The path length (l) is the physical. \[\log \left(. Beer Lambert Law E Value.
From www.slideserve.com
PPT Lab 6 Saliva Practical BeerLambert Law PowerPoint Presentation Beer Lambert Law E Value You can also use this calculator to. The path length (l) is the physical. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. A higher ε value means. Beer Lambert Law E Value.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law E Value The path length (l) is the physical. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. A higher ε value means stronger absorption at that wavelength. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. In most experiments, molar absorptivity (ε) and the length (b) are. Beer Lambert Law E Value.
From www.youtube.com
Introduction to UVVis Spectroscopy 03 BeerLambert Law YouTube Beer Lambert Law E Value Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The path length (l) is the physical. A higher ε value means. Beer Lambert Law E Value.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer Lambert Law E Value In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The path length (l) is the physical. A higher ε value means stronger absorption at that wavelength. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer Lambert Law E Value.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer Lambert Law E Value Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. The path length (l) is the physical. A higher ε value means. Beer Lambert Law E Value.
From www.youtube.com
BeerLambert law in easy way YouTube Beer Lambert Law E Value The path length (l) is the physical. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. A higher ε value means stronger absorption at that wavelength. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can. Beer Lambert Law E Value.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID Beer Lambert Law E Value You can also use this calculator to. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The path length (l) is. Beer Lambert Law E Value.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Beer Lambert Law E Value Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. A higher ε value means stronger absorption at that wavelength. The path. Beer Lambert Law E Value.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Beer Lambert Law E Value A higher ε value means stronger absorption at that wavelength. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The path length (l) is the physical. You can also use this calculator to. In most experiments, molar. Beer Lambert Law E Value.
From mungfali.com
UV Spectroscopy Beer Lambert Law Beer Lambert Law E Value In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The path length (l) is the physical. A higher ε value means stronger absorption at that wavelength. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer Lambert Law E Value.
From www.youtube.com
spectrum, UV Visible Spectroscopy, Beer Lambert’s Law Beer Lambert Law E Value \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the. A higher ε value means stronger absorption at that wavelength. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The path length (l) is the physical. In most experiments, molar absorptivity (ε) and the length (b) are. Beer Lambert Law E Value.