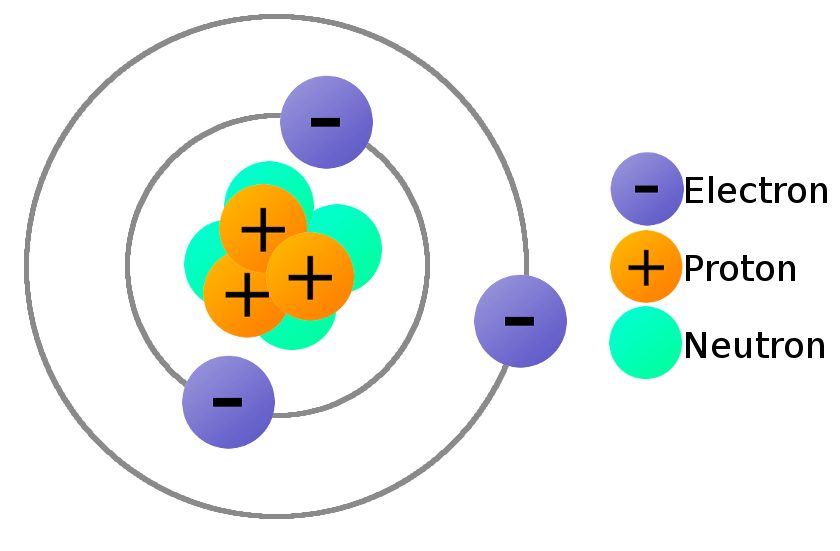
Do Atoms Have A Net Charge . Unlike protons and electrons, which. If it loses an electron, it becomes positively charged and is known as a cation. The number of protons of an atom cannot change via any. In their standard forms, elements have no net charge. Atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and negative charge, so. Charge is measured in coulombs (c), which represent 6.242×10 18 e If an atom has an equal number of protons and electrons, its net charge is 0. What is an atom (or group of atoms) that has an electric charge other than zero, and is created when an atom or group of atoms) gains or loses. If it gains an extra electron, it becomes negatively charged and is known as an anion. Of these three subatomic particle types, two (protons and electrons) carry a net electric charge, while neutrons are neutral and have no net. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation.
from learn.sparkfun.com
Charge is measured in coulombs (c), which represent 6.242×10 18 e Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. If an atom has an equal number of protons and electrons, its net charge is 0. Of these three subatomic particle types, two (protons and electrons) carry a net electric charge, while neutrons are neutral and have no net. If it loses an electron, it becomes positively charged and is known as a cation. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. The number of protons of an atom cannot change via any. Atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and negative charge, so. If it gains an extra electron, it becomes negatively charged and is known as an anion. In their standard forms, elements have no net charge.
What is Electricity? SparkFun Learn
Do Atoms Have A Net Charge If an atom has an equal number of protons and electrons, its net charge is 0. Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. Atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and negative charge, so. Charge is measured in coulombs (c), which represent 6.242×10 18 e If it gains an extra electron, it becomes negatively charged and is known as an anion. In their standard forms, elements have no net charge. The number of protons of an atom cannot change via any. Unlike protons and electrons, which. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Of these three subatomic particle types, two (protons and electrons) carry a net electric charge, while neutrons are neutral and have no net. If it loses an electron, it becomes positively charged and is known as a cation. What is an atom (or group of atoms) that has an electric charge other than zero, and is created when an atom or group of atoms) gains or loses. If an atom has an equal number of protons and electrons, its net charge is 0.
From telgurus.co.uk
What is the overall charge of the nucleus of an atom and why? Do Atoms Have A Net Charge What is an atom (or group of atoms) that has an electric charge other than zero, and is created when an atom or group of atoms) gains or loses. Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their. Do Atoms Have A Net Charge.
From www.expii.com
Neutrons — Structure & Properties Expii Do Atoms Have A Net Charge Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. If an atom has an equal number of protons and electrons, its net charge is 0. What is an atom (or group of atoms) that has an electric charge other than zero, and is created when an atom or group of. Do Atoms Have A Net Charge.
From www.numerade.com
SOLVED 2. Which of the following atoms has a net charge of+2 an atom Do Atoms Have A Net Charge Charge is measured in coulombs (c), which represent 6.242×10 18 e Of these three subatomic particle types, two (protons and electrons) carry a net electric charge, while neutrons are neutral and have no net. If an atom has an equal number of protons and electrons, its net charge is 0. What is an atom (or group of atoms) that has. Do Atoms Have A Net Charge.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Fields PowerPoint Do Atoms Have A Net Charge The number of protons of an atom cannot change via any. Of these three subatomic particle types, two (protons and electrons) carry a net electric charge, while neutrons are neutral and have no net. Unlike protons and electrons, which. Charge is measured in coulombs (c), which represent 6.242×10 18 e If it gains an extra electron, it becomes negatively charged. Do Atoms Have A Net Charge.
From www.toppr.com
45. An atom has net charge of 1. It has 18 electrons and 20 neutrons Do Atoms Have A Net Charge Unlike protons and electrons, which. Atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and negative charge, so. If it gains an extra electron, it becomes negatively charged and is known as an anion. In their standard forms, elements have no net charge. Charge is measured in coulombs (c), which represent. Do Atoms Have A Net Charge.
From slideplayer.com
Atomic structure Mrs. Meadows. ppt download Do Atoms Have A Net Charge Atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and negative charge, so. Of these three subatomic particle types, two (protons and electrons) carry a net electric charge, while neutrons are neutral and have no net. Charge is measured in coulombs (c), which represent 6.242×10 18 e In their standard forms,. Do Atoms Have A Net Charge.
From peyton-has-tapia.blogspot.com
An Atom With a Net Positive Charge Must Have More PeytonhasTapia Do Atoms Have A Net Charge What is an atom (or group of atoms) that has an electric charge other than zero, and is created when an atom or group of atoms) gains or loses. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. If it loses an electron, it becomes positively charged and is known as a cation. Charge is measured. Do Atoms Have A Net Charge.
From slideplayer.com
Chemistry for Biology Chapter 2 ppt download Do Atoms Have A Net Charge Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. Atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and negative charge, so. The number of protons of an atom cannot change via any. What is an atom (or group of atoms). Do Atoms Have A Net Charge.
From www.youtube.com
Calculating formal and net charge YouTube Do Atoms Have A Net Charge Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Charge is measured in coulombs (c), which represent 6.242×10 18 e If it gains an extra electron, it becomes negatively charged and is known as an anion. Unlike. Do Atoms Have A Net Charge.
From sciencenotes.org
Element Charges Chart How to Know the Charge of an Atom Do Atoms Have A Net Charge If an atom has an equal number of protons and electrons, its net charge is 0. If it gains an extra electron, it becomes negatively charged and is known as an anion. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Atoms, however, were known to be electrically neutral, which means that they carry the same. Do Atoms Have A Net Charge.
From www.slideserve.com
PPT Charge PowerPoint Presentation, free download ID1589402 Do Atoms Have A Net Charge If it loses an electron, it becomes positively charged and is known as a cation. The number of protons of an atom cannot change via any. If it gains an extra electron, it becomes negatively charged and is known as an anion. Neutrons do not have a net electric charge, so the number of neutrons does not matter in the. Do Atoms Have A Net Charge.
From www.slideserve.com
PPT Lecture 1 Charge and Coulomb’s Law PowerPoint Presentation, free Do Atoms Have A Net Charge Unlike protons and electrons, which. In their standard forms, elements have no net charge. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and negative charge, so. The number of protons of an atom cannot change via any.. Do Atoms Have A Net Charge.
From www.mrsciguy.com
Static Electricity Do Atoms Have A Net Charge The number of protons of an atom cannot change via any. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Of these three subatomic particle types, two (protons and electrons) carry a net electric charge, while neutrons are neutral and have no net. Neutrons do not have a net electric charge, so the number of neutrons. Do Atoms Have A Net Charge.
From classfullplymouth.z21.web.core.windows.net
Parts Of The Atom Do Atoms Have A Net Charge Of these three subatomic particle types, two (protons and electrons) carry a net electric charge, while neutrons are neutral and have no net. Unlike protons and electrons, which. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and. Do Atoms Have A Net Charge.
From www.youtube.com
Net Atomic Charges from the DDEC6 Method YouTube Do Atoms Have A Net Charge What is an atom (or group of atoms) that has an electric charge other than zero, and is created when an atom or group of atoms) gains or loses. Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. Charge is measured in coulombs (c), which represent 6.242×10 18 e If. Do Atoms Have A Net Charge.
From www.slideserve.com
PPT Physical Science PowerPoint Presentation, free download ID1589136 Do Atoms Have A Net Charge Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Charge is measured in coulombs (c), which represent 6.242×10 18 e If an atom has an equal number of protons and electrons, its net charge is 0. Atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and negative. Do Atoms Have A Net Charge.
From www.sliderbase.com
Atoms, molecules and ions Presentation Chemistry Do Atoms Have A Net Charge If an atom has an equal number of protons and electrons, its net charge is 0. Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. If it loses an electron, it becomes positively charged and is known as a cation. What is an atom (or group of atoms) that has. Do Atoms Have A Net Charge.
From studyrocket.co.uk
The Structure of the Atom GCSE Physics Science) AQA Do Atoms Have A Net Charge Of these three subatomic particle types, two (protons and electrons) carry a net electric charge, while neutrons are neutral and have no net. Charge is measured in coulombs (c), which represent 6.242×10 18 e What is an atom (or group of atoms) that has an electric charge other than zero, and is created when an atom or group of atoms). Do Atoms Have A Net Charge.
From www.youtube.com
WHAT IS NET CHARGE ON MOLECULE? YouTube Do Atoms Have A Net Charge Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. In their standard forms, elements have no net charge. If it gains an extra electron, it becomes negatively charged and is known as an anion. If it loses an electron, it becomes positively charged and is known as a cation. What. Do Atoms Have A Net Charge.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Do Atoms Have A Net Charge If it loses an electron, it becomes positively charged and is known as a cation. Unlike protons and electrons, which. Charge is measured in coulombs (c), which represent 6.242×10 18 e What is an atom (or group of atoms) that has an electric charge other than zero, and is created when an atom or group of atoms) gains or loses.. Do Atoms Have A Net Charge.
From www.youtube.com
What is Electric Charge and How Electricity Works Electronics Basics Do Atoms Have A Net Charge If it gains an extra electron, it becomes negatively charged and is known as an anion. Charge is measured in coulombs (c), which represent 6.242×10 18 e Unlike protons and electrons, which. The number of protons of an atom cannot change via any. What is an atom (or group of atoms) that has an electric charge other than zero, and. Do Atoms Have A Net Charge.
From www.chegg.com
Solved An Lewis structure is shown below. The Do Atoms Have A Net Charge Of these three subatomic particle types, two (protons and electrons) carry a net electric charge, while neutrons are neutral and have no net. Atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and negative charge, so. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. What is. Do Atoms Have A Net Charge.
From www.slideserve.com
PPT The Science of Water PowerPoint Presentation, free download ID Do Atoms Have A Net Charge Unlike protons and electrons, which. Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. What is an atom (or group of atoms) that has an electric charge other than zero, and is created when an atom or group of atoms) gains or loses. Of these three subatomic particle types, two. Do Atoms Have A Net Charge.
From www.slideserve.com
PPT Electric Field PowerPoint Presentation, free download ID1777746 Do Atoms Have A Net Charge Atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and negative charge, so. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. The number of protons of an atom cannot change via any. Charge is measured in coulombs (c), which represent 6.242×10 18 e Neutrons do not. Do Atoms Have A Net Charge.
From www.expii.com
Atoms — Definition & Overview Expii Do Atoms Have A Net Charge If an atom has an equal number of protons and electrons, its net charge is 0. Atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and negative charge, so. Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. The number of. Do Atoms Have A Net Charge.
From www.youtube.com
Atomic Number, Mass Number, and Net Charge YouTube Do Atoms Have A Net Charge In their standard forms, elements have no net charge. Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. Unlike protons and electrons, which. Charge is measured in coulombs (c), which represent 6.242×10 18 e The number of protons of an atom cannot change via any. If it loses an electron,. Do Atoms Have A Net Charge.
From www.slideserve.com
PPT The Atom PowerPoint Presentation, free download ID5056341 Do Atoms Have A Net Charge What is an atom (or group of atoms) that has an electric charge other than zero, and is created when an atom or group of atoms) gains or loses. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. The number of protons of an atom cannot change via any. If it loses an electron, it becomes. Do Atoms Have A Net Charge.
From hxegwcsmr.blob.core.windows.net
Do Atoms Have A Net Positive Electrical Charge at Ilda Vanbuskirk blog Do Atoms Have A Net Charge The number of protons of an atom cannot change via any. If it gains an extra electron, it becomes negatively charged and is known as an anion. What is an atom (or group of atoms) that has an electric charge other than zero, and is created when an atom or group of atoms) gains or loses. Unlike protons and electrons,. Do Atoms Have A Net Charge.
From electric.najura.my.id
An Electrically Charged Atom Is Called Do Atoms Have A Net Charge Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. The number of protons of an atom cannot change via any. Atoms, however, were known to be electrically neutral, which means that they carry the same amount of positive and negative charge, so. Charge is measured in coulombs (c), which represent. Do Atoms Have A Net Charge.
From www.youtube.com
Practice Problems Net Charge, Mass Number, Atomic Number YouTube Do Atoms Have A Net Charge If it gains an extra electron, it becomes negatively charged and is known as an anion. Charge is measured in coulombs (c), which represent 6.242×10 18 e The number of protons of an atom cannot change via any. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. In their standard forms, elements have no net charge.. Do Atoms Have A Net Charge.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation ID2508414 Do Atoms Have A Net Charge Of these three subatomic particle types, two (protons and electrons) carry a net electric charge, while neutrons are neutral and have no net. Charge is measured in coulombs (c), which represent 6.242×10 18 e Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Atoms, however, were known to be electrically neutral, which means that they carry. Do Atoms Have A Net Charge.
From www.numerade.com
SOLVED An Lewis structure is shown below.The structure only Do Atoms Have A Net Charge Charge is measured in coulombs (c), which represent 6.242×10 18 e Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. If it gains an extra electron, it becomes negatively charged and is known as an anion. If an atom has an equal number of protons and electrons, its net charge is 0. If it loses an. Do Atoms Have A Net Charge.
From www.numerade.com
SOLVED An Lewis structure shown below The structure only Do Atoms Have A Net Charge Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. If it gains an extra electron, it becomes negatively charged and is known as an anion. If an atom has an equal number of protons and electrons, its net charge is 0. Atoms, however, were known to be electrically neutral, which. Do Atoms Have A Net Charge.
From www.askiitians.com
Basic Properties of Electric Charge Study Material for IIT JEE Do Atoms Have A Net Charge If it gains an extra electron, it becomes negatively charged and is known as an anion. Unlike protons and electrons, which. The number of protons of an atom cannot change via any. Charge is measured in coulombs (c), which represent 6.242×10 18 e If it loses an electron, it becomes positively charged and is known as a cation. Atoms, however,. Do Atoms Have A Net Charge.
From hxegwcsmr.blob.core.windows.net
Do Atoms Have A Net Positive Electrical Charge at Ilda Vanbuskirk blog Do Atoms Have A Net Charge Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. If an atom has an equal number of protons and electrons, its net charge is 0. The number of protons of an atom cannot change via any. If it loses an electron, it becomes positively charged and is known as a. Do Atoms Have A Net Charge.