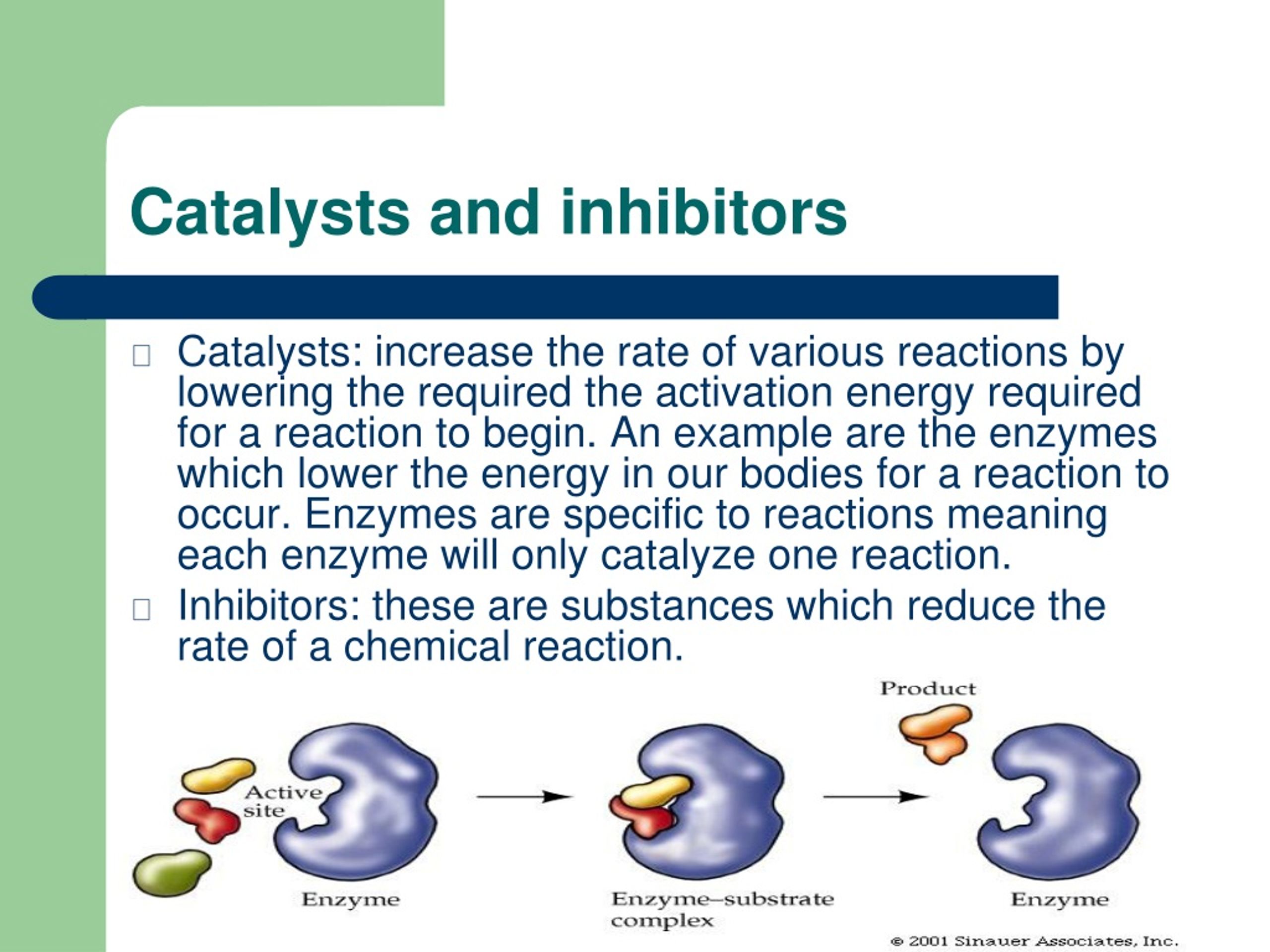
What Are Catalysts And Inhibitors . Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very little will happen. This lesson will give you a. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. If you light a match in that room (or just produce a spark), most of the. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. Catalysts increase reaction rates by. A catalyst is all about energy.
from www.slideserve.com
A catalyst is all about energy. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very little will happen. This lesson will give you a. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. In heterogeneous catalysis, catalysts provide a surface to. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. If you light a match in that room (or just produce a spark), most of the. Catalysts increase reaction rates by.
PPT Observing and describing chemical reactions PowerPoint
What Are Catalysts And Inhibitors If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very little will happen. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very little will happen. A catalyst is all about energy. If you light a match in that room (or just produce a spark), most of the. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. This lesson will give you a. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. In heterogeneous catalysis, catalysts provide a surface to. Catalysts increase reaction rates by.
From www.slideserve.com
PPT General Chemistry PowerPoint Presentation, free download ID1047081 What Are Catalysts And Inhibitors Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. This lesson will give you a. In heterogeneous catalysis, catalysts provide a surface to. Catalysis, the modification of the rate of a chemical reaction,. What Are Catalysts And Inhibitors.
From thecontentauthority.com
Catalyst vs Inhibitor Which Should You Use In Writing? What Are Catalysts And Inhibitors In heterogeneous catalysis, catalysts provide a surface to. If you light a match in that room (or just produce a spark), most of the. Catalysts increase reaction rates by. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very little will happen. Catalysis, the modification of the rate of a chemical reaction, usually an. What Are Catalysts And Inhibitors.
From www.youtube.com
Catalyst Promoter and Catalyst Inhibitor Chemistry YouTube What Are Catalysts And Inhibitors If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very little will happen. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. What Are Catalysts And Inhibitors.
From slideplayer.com
Click a hyperlink or folder tab to view the corresponding slides. ppt What Are Catalysts And Inhibitors Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. In heterogeneous catalysis, catalysts provide a surface to. If you light a match in that room (or just. What Are Catalysts And Inhibitors.
From differencebtw.com
Catalyst vs. Inhibitor Know the Difference What Are Catalysts And Inhibitors Catalysts increase reaction rates by. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is all about energy. This lesson will give you a. Catalysis, the modification of the rate. What Are Catalysts And Inhibitors.
From www.slideserve.com
PPT Observing and describing chemical reactions PowerPoint What Are Catalysts And Inhibitors While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is all about energy. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the. What Are Catalysts And Inhibitors.
From slideplayer.com
Chemical Reactions and Energy ppt download What Are Catalysts And Inhibitors Catalysts increase reaction rates by. This lesson will give you a. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Usually when someone refers to a catalyst,. What Are Catalysts And Inhibitors.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free What Are Catalysts And Inhibitors Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. If you light a match in that room (or just produce a spark), most of the. This lesson will give you a. A catalyst is all about energy. In heterogeneous catalysis, catalysts provide a surface to. If. What Are Catalysts And Inhibitors.
From www.youtube.com
Catalysts and Inhibitors Notes YouTube What Are Catalysts And Inhibitors This lesson will give you a. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. Catalysis, the modification of the rate of a chemical reaction,. What Are Catalysts And Inhibitors.
From ablogwithadifference.com
Catalyst and Inhibitor The best 5 difference What Are Catalysts And Inhibitors This lesson will give you a. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. In heterogeneous catalysis, catalysts provide a surface to. Catalysts increase reaction rates by. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is all about energy. Catalysts. What Are Catalysts And Inhibitors.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii What Are Catalysts And Inhibitors Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is all about energy. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. This lesson will give. What Are Catalysts And Inhibitors.
From slideplayer.com
Equilibrium Chapter ppt download What Are Catalysts And Inhibitors In heterogeneous catalysis, catalysts provide a surface to. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. Catalysts increase reaction rates by. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. If you light a match in that room (or. What Are Catalysts And Inhibitors.
From slideplayer.com
Click a hyperlink or folder tab to view the corresponding slides. ppt What Are Catalysts And Inhibitors Catalysts increase reaction rates by. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. If you light a match in that room (or just produce a spark), most of the. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical. What Are Catalysts And Inhibitors.
From slideplayer.com
Click a hyperlink or folder tab to view the corresponding slides. ppt What Are Catalysts And Inhibitors Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. This lesson will give you a. If you light a match in that room (or just produce a spark), most of the. In heterogeneous catalysis, catalysts provide a surface to. Catalysts play an essential role in our modern industrial economy, in. What Are Catalysts And Inhibitors.
From www.youtube.com
Catalyst and Inhibitors YouTube What Are Catalysts And Inhibitors Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very little will happen. If you light a match in that room (or just produce a. What Are Catalysts And Inhibitors.
From slideplayer.com
ppt download What Are Catalysts And Inhibitors Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. This lesson will give you a. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. Catalysts allow a reaction to proceed via a pathway that. What Are Catalysts And Inhibitors.
From www.youtube.com
Catalysts and Inhibitors (chemical part 81 for CBSE class 12 What Are Catalysts And Inhibitors Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. This lesson will give you a. Catalysts allow a reaction to proceed via a pathway that. What Are Catalysts And Inhibitors.
From www.askdifference.com
Catalyst vs. Inhibitor — What’s the Difference? What Are Catalysts And Inhibitors A catalyst is all about energy. Catalysts increase reaction rates by. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very little will happen. This lesson will give you a. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis, the modification of the. What Are Catalysts And Inhibitors.
From slideplayer.com
Introduction to Chemistry Chapter ppt download What Are Catalysts And Inhibitors If you light a match in that room (or just produce a spark), most of the. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. A catalyst is all about energy. In heterogeneous catalysis, catalysts provide a surface to. Catalysts. What Are Catalysts And Inhibitors.
From www.slideserve.com
PPT Catalysts and inhibitors, But Mostly Catalysts PowerPoint What Are Catalysts And Inhibitors Catalysts increase reaction rates by. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. If you light a match in that room (or just produce a spark),. What Are Catalysts And Inhibitors.
From slideplayer.com
Chemical Reactions Fireworks are a result of chemical reactions. ppt What Are Catalysts And Inhibitors Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is all about energy. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very little will happen. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst. What Are Catalysts And Inhibitors.
From slideplayer.com
Chemical Reactions Test on Friday April ppt download What Are Catalysts And Inhibitors Catalysts increase reaction rates by. A catalyst is all about energy. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. If you light a match in that room (or just produce a spark), most of. What Are Catalysts And Inhibitors.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry What Are Catalysts And Inhibitors If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very little will happen. This lesson will give you a. A catalyst is all about energy. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. Catalysts allow a reaction to proceed via a. What Are Catalysts And Inhibitors.
From slideplayer.com
Ch 21 Chemical Changes Bellwork ppt download What Are Catalysts And Inhibitors If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very little will happen. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. In heterogeneous catalysis, catalysts provide a surface to. This lesson will give you a. Catalysts increase reaction rates. What Are Catalysts And Inhibitors.
From www.slideserve.com
PPT Catalysts and inhibitors, But Mostly Catalysts PowerPoint What Are Catalysts And Inhibitors In heterogeneous catalysis, catalysts provide a surface to. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. Catalysts increase reaction rates by. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance. What Are Catalysts And Inhibitors.
From www.youtube.com
Catalysts and Inhibitors YouTube What Are Catalysts And Inhibitors A catalyst is all about energy. If you light a match in that room (or just produce a spark), most of the. Catalysts increase reaction rates by. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. This lesson will give. What Are Catalysts And Inhibitors.
From slideplayer.com
ppt download What Are Catalysts And Inhibitors Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. This lesson will give you a. A catalyst is all about energy. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation.. What Are Catalysts And Inhibitors.
From www.slideserve.com
PPT & Equilibrium PowerPoint Presentation, free download What Are Catalysts And Inhibitors Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. Catalysts play an essential role in our modern industrial economy, in our stewardship of the environment, and in all biological processes. This. What Are Catalysts And Inhibitors.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID What Are Catalysts And Inhibitors Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. If you fill a room with hydrogen gas (h 2) and oxygen. What Are Catalysts And Inhibitors.
From www.slideserve.com
PPT Science 9 Unit B Matter and Chemical Change PowerPoint What Are Catalysts And Inhibitors This lesson will give you a. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is all about energy. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Usually when someone refers to a. What Are Catalysts And Inhibitors.
From www.youtube.com
B.7.2 Compare catalysts and biological catalysts (enzymes What Are Catalysts And Inhibitors This lesson will give you a. If you light a match in that room (or just produce a spark), most of the. In heterogeneous catalysis, catalysts provide a surface to. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very little will happen. Catalysts allow a reaction to proceed via a pathway that has. What Are Catalysts And Inhibitors.
From teachmephysiology.com
Enzyme Inhibition Types of Inhibition Allosteric Regulation What Are Catalysts And Inhibitors If you light a match in that room (or just produce a spark), most of the. This lesson will give you a. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts play an essential role in our modern industrial economy, in our stewardship of the. What Are Catalysts And Inhibitors.
From www.slideserve.com
PPT Chapter 21 Section 4 PowerPoint Presentation, free download ID What Are Catalysts And Inhibitors If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very little will happen. This lesson will give you a. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. A catalyst is all about energy. If you light a match in. What Are Catalysts And Inhibitors.
From www.slideserve.com
PPT Catalysts and inhibitors, But Mostly Catalysts PowerPoint What Are Catalysts And Inhibitors In heterogeneous catalysis, catalysts provide a surface to. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. This lesson will give you a. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. If you. What Are Catalysts And Inhibitors.
From www.differencebetween.com
Difference Between Catalyst and Inhibitor Compare the Difference What Are Catalysts And Inhibitors In heterogeneous catalysis, catalysts provide a surface to. Usually when someone refers to a catalyst, they mean a positive catalyst, which is a catalyst that speeds up the rate of a chemical reaction by lowering its activation. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction.. What Are Catalysts And Inhibitors.