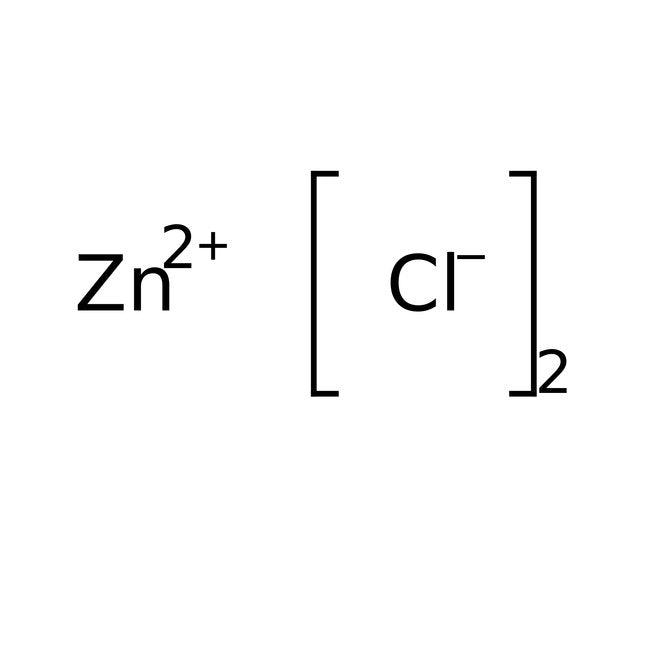Zinc Chloride Ionic Or Covalent . Predict the type of compound formed from elements based on their location within the periodic table. Zinc chloride is an inorganic binary salt. The first question we ask is if the compound is ionic or covalent? How to draw the zncl 2 structure? Define ionic and molecular (covalent) compounds. To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. That is, does it have ionic bonds, or covalent bonds? Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly. The lewis structure of zncl 2 can give us a clear idea of the molecular property. The structure of a zncl 2 molecule is illustrated below.
from sciencelab.co.ke
The lewis structure of zncl 2 can give us a clear idea of the molecular property. Zinc chloride is an inorganic binary salt. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Ionic compounds generally form from metals and nonmetals. The structure of a zncl 2 molecule is illustrated below. To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. The first question we ask is if the compound is ionic or covalent? How to draw the zncl 2 structure? Define ionic and molecular (covalent) compounds. That is, does it have ionic bonds, or covalent bonds?
Zinc Chloride Sciencelab limited
Zinc Chloride Ionic Or Covalent Compounds that do not contain ions, but instead consist of atoms bonded tightly. Predict the type of compound formed from elements based on their location within the periodic table. Compounds that do not contain ions, but instead consist of atoms bonded tightly. The first question we ask is if the compound is ionic or covalent? Ionic compounds generally form from metals and nonmetals. To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. How to draw the zncl 2 structure? The lewis structure of zncl 2 can give us a clear idea of the molecular property. That is, does it have ionic bonds, or covalent bonds? The structure of a zncl 2 molecule is illustrated below. Define ionic and molecular (covalent) compounds. Zinc chloride is an inorganic binary salt.
From www.youtube.com
Lewis Structure of ZnCl2 (zinc chloride) YouTube Zinc Chloride Ionic Or Covalent Define ionic and molecular (covalent) compounds. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Ionic compounds generally form from metals and nonmetals. The lewis structure of zncl 2 can give us a clear idea of the molecular property. How to draw the zncl 2 structure? The structure of a zncl 2 molecule is illustrated below.. Zinc Chloride Ionic Or Covalent.
From www.slideserve.com
PPT Net Ionic in SR PowerPoint Presentation, free download ID5979486 Zinc Chloride Ionic Or Covalent To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. Define ionic and molecular (covalent) compounds. That is, does it have ionic bonds, or covalent bonds? Predict the type of compound formed from elements based on their location within the periodic table. How to draw the zncl 2 structure? The lewis structure of. Zinc Chloride Ionic Or Covalent.
From exoquepbq.blob.core.windows.net
Potassium Sulfide Zinc Chloride at Gerry Muniz blog Zinc Chloride Ionic Or Covalent The lewis structure of zncl 2 can give us a clear idea of the molecular property. To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. Define ionic and molecular (covalent) compounds. That is, does it have ionic bonds, or covalent bonds? How to draw the zncl 2 structure? Predict the type of. Zinc Chloride Ionic Or Covalent.
From www.youtube.com
How to Write the Net Ionic Equation for ZnCl2 + NaOH = Zn(OH)2 + NaCl Zinc Chloride Ionic Or Covalent The structure of a zncl 2 molecule is illustrated below. The first question we ask is if the compound is ionic or covalent? Compounds that do not contain ions, but instead consist of atoms bonded tightly. Zinc chloride is an inorganic binary salt. To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the.. Zinc Chloride Ionic Or Covalent.
From www.youtube.com
How to Balance Zn + CuCl2 = ZnCl2 + Cu Zinc + Copper (II) chloride Zinc Chloride Ionic Or Covalent Ionic compounds generally form from metals and nonmetals. Predict the type of compound formed from elements based on their location within the periodic table. The structure of a zncl 2 molecule is illustrated below. To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. How to draw the zncl 2 structure? The lewis. Zinc Chloride Ionic Or Covalent.
From www.numerade.com
SOLVED 'Identify the ionic and covalent bond in the following Zinc Chloride Ionic Or Covalent The lewis structure of zncl 2 can give us a clear idea of the molecular property. Ionic compounds generally form from metals and nonmetals. Predict the type of compound formed from elements based on their location within the periodic table. Compounds that do not contain ions, but instead consist of atoms bonded tightly. How to draw the zncl 2 structure?. Zinc Chloride Ionic Or Covalent.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Zinc Chloride Ionic Or Covalent The lewis structure of zncl 2 can give us a clear idea of the molecular property. The first question we ask is if the compound is ionic or covalent? That is, does it have ionic bonds, or covalent bonds? Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly.. Zinc Chloride Ionic Or Covalent.
From chemistrylearnwithsangam.blogspot.com
chemistry knowledge Comparison between Covalent and Ionic Bond Zinc Chloride Ionic Or Covalent Compounds that do not contain ions, but instead consist of atoms bonded tightly. How to draw the zncl 2 structure? To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. Ionic compounds generally form from metals and nonmetals. The lewis structure of zncl 2 can give us a clear idea of the molecular. Zinc Chloride Ionic Or Covalent.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Zinc Chloride Ionic Or Covalent Zinc chloride is an inorganic binary salt. That is, does it have ionic bonds, or covalent bonds? Compounds that do not contain ions, but instead consist of atoms bonded tightly. Define ionic and molecular (covalent) compounds. The first question we ask is if the compound is ionic or covalent? The structure of a zncl 2 molecule is illustrated below. To. Zinc Chloride Ionic Or Covalent.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Zinc Chloride Ionic Or Covalent Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly. That is, does it have ionic bonds, or covalent bonds? Define ionic and molecular (covalent) compounds. The structure of a zncl 2 molecule is illustrated below. To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular). Zinc Chloride Ionic Or Covalent.
From www.researchgate.net
Schematic representation of formation of both ionic and covalent Zinc Chloride Ionic Or Covalent That is, does it have ionic bonds, or covalent bonds? Define ionic and molecular (covalent) compounds. The first question we ask is if the compound is ionic or covalent? How to draw the zncl 2 structure? Predict the type of compound formed from elements based on their location within the periodic table. The lewis structure of zncl 2 can give. Zinc Chloride Ionic Or Covalent.
From www.youtube.com
How to Write the Formula for Zinc chloride (ZnCl2) YouTube Zinc Chloride Ionic Or Covalent The structure of a zncl 2 molecule is illustrated below. Define ionic and molecular (covalent) compounds. Predict the type of compound formed from elements based on their location within the periodic table. Ionic compounds generally form from metals and nonmetals. To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. How to draw. Zinc Chloride Ionic Or Covalent.
From www.shutterstock.com
122 Zinc Chloride Images, Stock Photos & Vectors Shutterstock Zinc Chloride Ionic Or Covalent To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. Predict the type of compound formed from elements based on their location within the periodic table. Define ionic and molecular (covalent) compounds. Compounds that do not contain ions, but instead consist of atoms bonded tightly. The first question we ask is if the. Zinc Chloride Ionic Or Covalent.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Zinc Chloride Ionic Or Covalent The first question we ask is if the compound is ionic or covalent? The structure of a zncl 2 molecule is illustrated below. Zinc chloride is an inorganic binary salt. Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly. That is, does it have ionic bonds, or covalent. Zinc Chloride Ionic Or Covalent.
From www.youtube.com
Equation for ZnCl2 + H2O (Zinc chloride + Water) YouTube Zinc Chloride Ionic Or Covalent Compounds that do not contain ions, but instead consist of atoms bonded tightly. To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. How to draw the zncl 2 structure? The lewis structure of zncl 2 can give us a clear idea of the molecular property. Define ionic and molecular (covalent) compounds. Predict. Zinc Chloride Ionic Or Covalent.
From express.adobe.com
Zinc and Copper Chloride Zinc Chloride Ionic Or Covalent The structure of a zncl 2 molecule is illustrated below. Zinc chloride is an inorganic binary salt. Predict the type of compound formed from elements based on their location within the periodic table. How to draw the zncl 2 structure? Define ionic and molecular (covalent) compounds. The first question we ask is if the compound is ionic or covalent? Compounds. Zinc Chloride Ionic Or Covalent.
From www.youtube.com
Is ZnCl2 (Zinc chloride) Ionic or Covalent? YouTube Zinc Chloride Ionic Or Covalent That is, does it have ionic bonds, or covalent bonds? How to draw the zncl 2 structure? Compounds that do not contain ions, but instead consist of atoms bonded tightly. The lewis structure of zncl 2 can give us a clear idea of the molecular property. Ionic compounds generally form from metals and nonmetals. The structure of a zncl 2. Zinc Chloride Ionic Or Covalent.
From sciencelab.co.ke
Zinc Chloride Sciencelab limited Zinc Chloride Ionic Or Covalent Ionic compounds generally form from metals and nonmetals. Define ionic and molecular (covalent) compounds. How to draw the zncl 2 structure? Zinc chloride is an inorganic binary salt. The first question we ask is if the compound is ionic or covalent? Predict the type of compound formed from elements based on their location within the periodic table. That is, does. Zinc Chloride Ionic Or Covalent.
From www.slideserve.com
PPT Recap Atomic Structure PowerPoint Presentation, free download Zinc Chloride Ionic Or Covalent The lewis structure of zncl 2 can give us a clear idea of the molecular property. How to draw the zncl 2 structure? Ionic compounds generally form from metals and nonmetals. The structure of a zncl 2 molecule is illustrated below. Define ionic and molecular (covalent) compounds. Compounds that do not contain ions, but instead consist of atoms bonded tightly.. Zinc Chloride Ionic Or Covalent.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Zinc Chloride Ionic Or Covalent The first question we ask is if the compound is ionic or covalent? Define ionic and molecular (covalent) compounds. Ionic compounds generally form from metals and nonmetals. The lewis structure of zncl 2 can give us a clear idea of the molecular property. The structure of a zncl 2 molecule is illustrated below. That is, does it have ionic bonds,. Zinc Chloride Ionic Or Covalent.
From childhealthpolicy.vumc.org
🌱 What is the chemical formula of zinc chloride. What is the chemical Zinc Chloride Ionic Or Covalent Compounds that do not contain ions, but instead consist of atoms bonded tightly. Predict the type of compound formed from elements based on their location within the periodic table. How to draw the zncl 2 structure? Define ionic and molecular (covalent) compounds. Ionic compounds generally form from metals and nonmetals. The first question we ask is if the compound is. Zinc Chloride Ionic Or Covalent.
From cartoondealer.com
Ionic Compound Cubic Crystal Structure Cartoon Vector CartoonDealer Zinc Chloride Ionic Or Covalent Define ionic and molecular (covalent) compounds. To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. That is, does it have ionic bonds, or covalent bonds? Zinc chloride is an inorganic binary salt. Predict the type of compound formed from elements based on their location within the periodic table. The lewis structure of. Zinc Chloride Ionic Or Covalent.
From fourthgradegc.blogspot.com
Fourth Grade GC August 2013 Zinc Chloride Ionic Or Covalent That is, does it have ionic bonds, or covalent bonds? How to draw the zncl 2 structure? Define ionic and molecular (covalent) compounds. The structure of a zncl 2 molecule is illustrated below. Ionic compounds generally form from metals and nonmetals. The first question we ask is if the compound is ionic or covalent? To tell if zncl2 (zinc chloride). Zinc Chloride Ionic Or Covalent.
From www.fishersci.co.uk
Zinc Chloride solution 0.1mol/L, Fisher Chemical Fisher Scientific Zinc Chloride Ionic Or Covalent Ionic compounds generally form from metals and nonmetals. How to draw the zncl 2 structure? The structure of a zncl 2 molecule is illustrated below. Zinc chloride is an inorganic binary salt. The lewis structure of zncl 2 can give us a clear idea of the molecular property. Define ionic and molecular (covalent) compounds. That is, does it have ionic. Zinc Chloride Ionic Or Covalent.
From www.sunshinetrading.co
Zinc Chloride Sunshine Trading Company Zinc Chloride Ionic Or Covalent The first question we ask is if the compound is ionic or covalent? The structure of a zncl 2 molecule is illustrated below. Define ionic and molecular (covalent) compounds. Ionic compounds generally form from metals and nonmetals. The lewis structure of zncl 2 can give us a clear idea of the molecular property. How to draw the zncl 2 structure?. Zinc Chloride Ionic Or Covalent.
From www.pw.live
Zinc Chloride Formula Zinc Chloride Ionic Or Covalent To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. Zinc chloride is an inorganic binary salt. The first question we ask is if the compound is ionic or covalent? The lewis structure of zncl 2 can give us a clear idea of the molecular property. Compounds that do not contain ions, but. Zinc Chloride Ionic Or Covalent.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + HCl = ZnCl2 + H2 YouTube Zinc Chloride Ionic Or Covalent Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Define ionic and molecular (covalent) compounds. The lewis structure of zncl 2 can give us a clear idea of the molecular property. Predict the type of compound formed from elements based on their location within the periodic table. To. Zinc Chloride Ionic Or Covalent.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Zinc Chloride Ionic Or Covalent The structure of a zncl 2 molecule is illustrated below. The lewis structure of zncl 2 can give us a clear idea of the molecular property. Zinc chloride is an inorganic binary salt. To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. The first question we ask is if the compound is. Zinc Chloride Ionic Or Covalent.
From studylib.net
Ionic & Covalent Nomenclature PowerPoint Zinc Chloride Ionic Or Covalent The lewis structure of zncl 2 can give us a clear idea of the molecular property. To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Define ionic and molecular (covalent) compounds. That is, does it have ionic bonds, or. Zinc Chloride Ionic Or Covalent.
From www.youtube.com
Is ZnF2 Ionic or Covalent/Molecular? YouTube Zinc Chloride Ionic Or Covalent The structure of a zncl 2 molecule is illustrated below. That is, does it have ionic bonds, or covalent bonds? The first question we ask is if the compound is ionic or covalent? Predict the type of compound formed from elements based on their location within the periodic table. How to draw the zncl 2 structure? Compounds that do not. Zinc Chloride Ionic Or Covalent.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Zinc Chloride Ionic Or Covalent Ionic compounds generally form from metals and nonmetals. Define ionic and molecular (covalent) compounds. How to draw the zncl 2 structure? To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. Zinc chloride is an inorganic binary salt. The lewis structure of zncl 2 can give us a clear idea of the molecular. Zinc Chloride Ionic Or Covalent.
From www.slideserve.com
PPT C/W PowerPoint Presentation, free download ID2216107 Zinc Chloride Ionic Or Covalent The lewis structure of zncl 2 can give us a clear idea of the molecular property. Compounds that do not contain ions, but instead consist of atoms bonded tightly. Define ionic and molecular (covalent) compounds. Zinc chloride is an inorganic binary salt. To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. Ionic. Zinc Chloride Ionic Or Covalent.
From www.pinterest.com
Learn the difference between ionic and covalent bonds. See examples of Zinc Chloride Ionic Or Covalent How to draw the zncl 2 structure? Define ionic and molecular (covalent) compounds. The first question we ask is if the compound is ionic or covalent? The lewis structure of zncl 2 can give us a clear idea of the molecular property. Predict the type of compound formed from elements based on their location within the periodic table. Zinc chloride. Zinc Chloride Ionic Or Covalent.
From sciencetrends.com
What Is The Ionic Charge Of Zinc (Zn)? Science Trends Zinc Chloride Ionic Or Covalent The structure of a zncl 2 molecule is illustrated below. Zinc chloride is an inorganic binary salt. How to draw the zncl 2 structure? Compounds that do not contain ions, but instead consist of atoms bonded tightly. Ionic compounds generally form from metals and nonmetals. Define ionic and molecular (covalent) compounds. The lewis structure of zncl 2 can give us. Zinc Chloride Ionic Or Covalent.
From www.chemistrylearner.com
Ionic, Covalent, and Metallic Bonds Differences and Similarities Zinc Chloride Ionic Or Covalent That is, does it have ionic bonds, or covalent bonds? To tell if zncl2 (zinc chloride) is ionic or covalent (also called molecular) we look at the. The structure of a zncl 2 molecule is illustrated below. The lewis structure of zncl 2 can give us a clear idea of the molecular property. Zinc chloride is an inorganic binary salt.. Zinc Chloride Ionic Or Covalent.