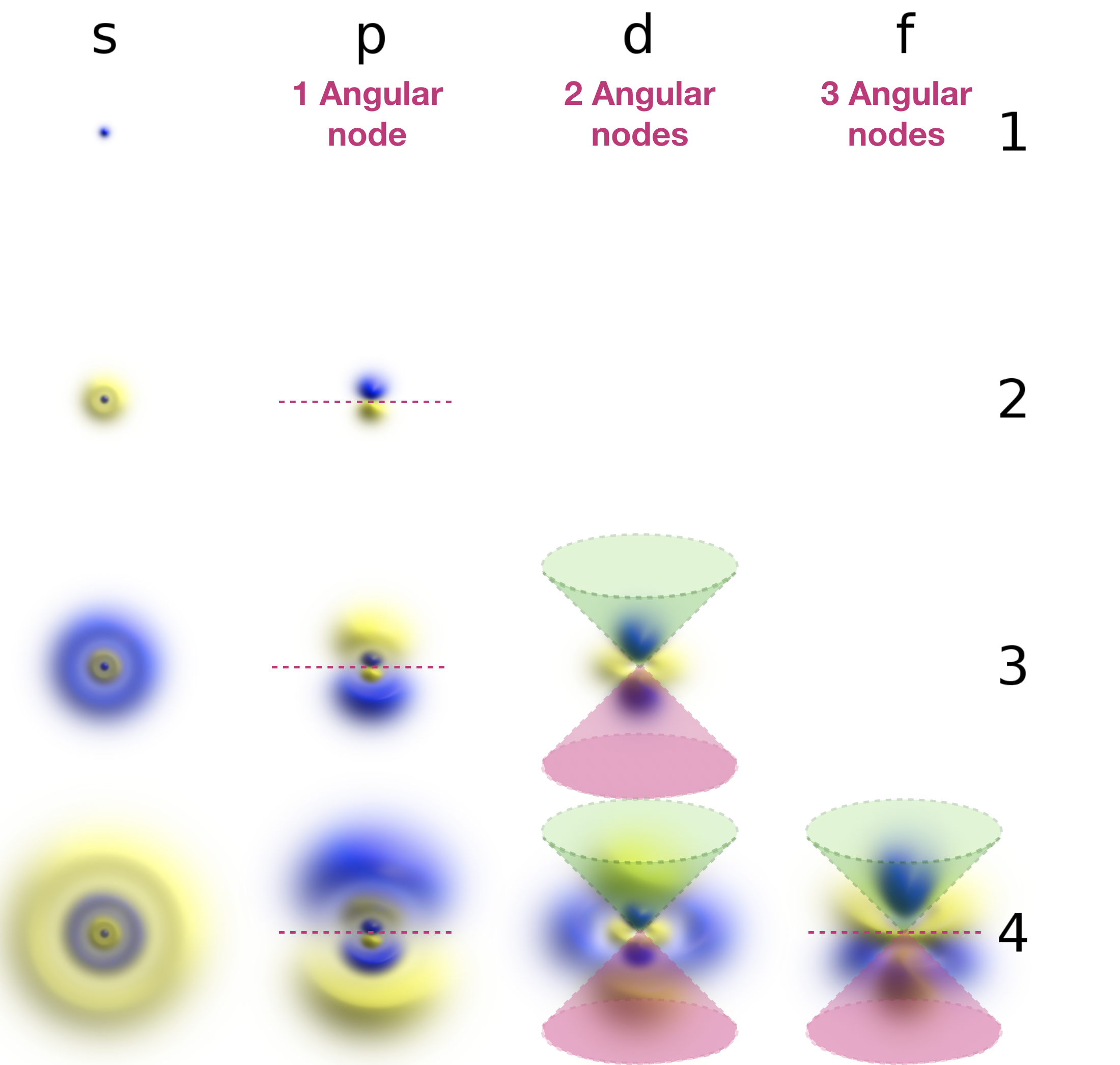
Spherical Node Mean . If $r(r_1) = 0$, there exists a radial node. The radial node is a sphere with radius $r_1$. Therefore the terms radial node and spherical node are the same. Nodes are regions in atoms where no electron can ever be found. A radial node is a sphere (rather than an angular node which is a flat plane) that occurs when the radial wavefunction for an atomic orbital is equal to zero or. The minima correspond to spherical nodes (regions of zero electron probability), which alternate with spherical regions of nonzero. Radial nodes are nodes inside the orbital lobes as far as i can understand. There are two types of nodes as radial nodes and angular nodes. A nodal surface is also called a radial node, which is a hollow spherical region in which electrons cannot be. A nodal plane is also called an angular node, which is either a plane. The main difference between radial. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals.
from chem.libretexts.org
A radial node is a sphere (rather than an angular node which is a flat plane) that occurs when the radial wavefunction for an atomic orbital is equal to zero or. Therefore the terms radial node and spherical node are the same. If $r(r_1) = 0$, there exists a radial node. The main difference between radial. There are two types of nodes as radial nodes and angular nodes. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. A nodal surface is also called a radial node, which is a hollow spherical region in which electrons cannot be. Radial nodes are nodes inside the orbital lobes as far as i can understand. Nodes are regions in atoms where no electron can ever be found. A nodal plane is also called an angular node, which is either a plane.
0.1.1 Review of Orbital Shapes Chemistry LibreTexts
Spherical Node Mean A nodal surface is also called a radial node, which is a hollow spherical region in which electrons cannot be. There are two types of nodes as radial nodes and angular nodes. A nodal plane is also called an angular node, which is either a plane. Nodes are regions in atoms where no electron can ever be found. Radial nodes are nodes inside the orbital lobes as far as i can understand. A radial node is a sphere (rather than an angular node which is a flat plane) that occurs when the radial wavefunction for an atomic orbital is equal to zero or. If $r(r_1) = 0$, there exists a radial node. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. Therefore the terms radial node and spherical node are the same. The main difference between radial. The minima correspond to spherical nodes (regions of zero electron probability), which alternate with spherical regions of nonzero. The radial node is a sphere with radius $r_1$. A nodal surface is also called a radial node, which is a hollow spherical region in which electrons cannot be.
From www.youtube.com
Spherical coordinate system YouTube Spherical Node Mean Nodes are regions in atoms where no electron can ever be found. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. Therefore the terms radial node and spherical node are the same. There are two types of nodes as radial nodes and angular nodes. Radial nodes are nodes inside. Spherical Node Mean.
From www.researchgate.net
Node division under the spherical coordinate system. Download Spherical Node Mean The minima correspond to spherical nodes (regions of zero electron probability), which alternate with spherical regions of nonzero. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. The radial node is a sphere with radius $r_1$. Radial nodes are nodes inside the orbital lobes as far as i can. Spherical Node Mean.
From www.researchgate.net
The concentric spheres are the basis of the Spherical Layout Spherical Node Mean Therefore the terms radial node and spherical node are the same. The minima correspond to spherical nodes (regions of zero electron probability), which alternate with spherical regions of nonzero. If $r(r_1) = 0$, there exists a radial node. The radial node is a sphere with radius $r_1$. There are two types of nodes as radial nodes and angular nodes. Radial. Spherical Node Mean.
From www.researchgate.net
Schematic of scratching process with a spherical indenter and stress Spherical Node Mean A radial node is a sphere (rather than an angular node which is a flat plane) that occurs when the radial wavefunction for an atomic orbital is equal to zero or. The radial node is a sphere with radius $r_1$. If $r(r_1) = 0$, there exists a radial node. Analogically, a constant angle of angular nodes leads to a 3d. Spherical Node Mean.
From www.vrogue.co
What Is The Difference Between Angular Node And Spher vrogue.co Spherical Node Mean The minima correspond to spherical nodes (regions of zero electron probability), which alternate with spherical regions of nonzero. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. The radial node is a sphere with radius $r_1$. There are two types of nodes as radial nodes and angular nodes. A. Spherical Node Mean.
From www.slideserve.com
PPT Atomic Line Emission Spectra and Niels Bohr PowerPoint Spherical Node Mean Nodes are regions in atoms where no electron can ever be found. There are two types of nodes as radial nodes and angular nodes. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. Therefore the terms radial node and spherical node are the same. The minima correspond to spherical. Spherical Node Mean.
From www.theochem.ru.nl
Spherical harmonics Knowino Spherical Node Mean Nodes are regions in atoms where no electron can ever be found. The main difference between radial. A nodal plane is also called an angular node, which is either a plane. A radial node is a sphere (rather than an angular node which is a flat plane) that occurs when the radial wavefunction for an atomic orbital is equal to. Spherical Node Mean.
From www.vrogue.co
What Is The Difference Between Angular Node And Spher vrogue.co Spherical Node Mean If $r(r_1) = 0$, there exists a radial node. The minima correspond to spherical nodes (regions of zero electron probability), which alternate with spherical regions of nonzero. A nodal plane is also called an angular node, which is either a plane. The main difference between radial. Therefore the terms radial node and spherical node are the same. Radial nodes are. Spherical Node Mean.
From www.slideserve.com
PPT Review Chapter 8 The Quantum Mechanical Atom PowerPoint Spherical Node Mean There are two types of nodes as radial nodes and angular nodes. The radial node is a sphere with radius $r_1$. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. The main difference between radial. The minima correspond to spherical nodes (regions of zero electron probability), which alternate with. Spherical Node Mean.
From www.britannica.com
Sorbital physics Britannica Spherical Node Mean A nodal surface is also called a radial node, which is a hollow spherical region in which electrons cannot be. The minima correspond to spherical nodes (regions of zero electron probability), which alternate with spherical regions of nonzero. If $r(r_1) = 0$, there exists a radial node. Nodes are regions in atoms where no electron can ever be found. Therefore. Spherical Node Mean.
From www.researchgate.net
Node division of spherical coal particles. Download Scientific Diagram Spherical Node Mean If $r(r_1) = 0$, there exists a radial node. The minima correspond to spherical nodes (regions of zero electron probability), which alternate with spherical regions of nonzero. A nodal plane is also called an angular node, which is either a plane. A nodal surface is also called a radial node, which is a hollow spherical region in which electrons cannot. Spherical Node Mean.
From www.researchgate.net
Quadrature nodes on a spherical triangle lifted from the projected Spherical Node Mean A nodal plane is also called an angular node, which is either a plane. Radial nodes are nodes inside the orbital lobes as far as i can understand. The radial node is a sphere with radius $r_1$. A nodal surface is also called a radial node, which is a hollow spherical region in which electrons cannot be. Analogically, a constant. Spherical Node Mean.
From classnotes.org.in
Shapes of Atomic Orbital Chemistry, Class 11, Structure of Atom Spherical Node Mean The main difference between radial. A nodal surface is also called a radial node, which is a hollow spherical region in which electrons cannot be. If $r(r_1) = 0$, there exists a radial node. Nodes are regions in atoms where no electron can ever be found. Therefore the terms radial node and spherical node are the same. A radial node. Spherical Node Mean.
From www.reddit.com
What is a node and why is there a zero probability of finding an Spherical Node Mean There are two types of nodes as radial nodes and angular nodes. The radial node is a sphere with radius $r_1$. A nodal plane is also called an angular node, which is either a plane. If $r(r_1) = 0$, there exists a radial node. Therefore the terms radial node and spherical node are the same. The main difference between radial.. Spherical Node Mean.
From www.reddit.com
How do I identify the radial node and angular nodes on this 4d orbital Spherical Node Mean Nodes are regions in atoms where no electron can ever be found. A nodal plane is also called an angular node, which is either a plane. If $r(r_1) = 0$, there exists a radial node. A radial node is a sphere (rather than an angular node which is a flat plane) that occurs when the radial wavefunction for an atomic. Spherical Node Mean.
From www.gkseries.com
The number of spherical nodes in 3p orbitals are Spherical Node Mean If $r(r_1) = 0$, there exists a radial node. A nodal surface is also called a radial node, which is a hollow spherical region in which electrons cannot be. The main difference between radial. The radial node is a sphere with radius $r_1$. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like. Spherical Node Mean.
From www.slideserve.com
PPT wavelength PowerPoint Presentation, free download ID5146419 Spherical Node Mean The minima correspond to spherical nodes (regions of zero electron probability), which alternate with spherical regions of nonzero. Therefore the terms radial node and spherical node are the same. The radial node is a sphere with radius $r_1$. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. Radial nodes. Spherical Node Mean.
From www.chegg.com
Solved What is the relationship between n, ?, and the total Spherical Node Mean Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. The radial node is a sphere with radius $r_1$. A nodal plane is also called an angular node, which is either a plane. A nodal surface is also called a radial node, which is a hollow spherical region in which. Spherical Node Mean.
From jayaretv.com
Spherical Camera Node JayAreTV Spherical Node Mean Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. The radial node is a sphere with radius $r_1$. There are two types of nodes as radial nodes and angular nodes. The main difference between radial. A nodal plane is also called an angular node, which is either a plane.. Spherical Node Mean.
From chem.libretexts.org
D Spherical Coordinates Chemistry LibreTexts Spherical Node Mean Radial nodes are nodes inside the orbital lobes as far as i can understand. A radial node is a sphere (rather than an angular node which is a flat plane) that occurs when the radial wavefunction for an atomic orbital is equal to zero or. There are two types of nodes as radial nodes and angular nodes. The radial node. Spherical Node Mean.
From www.researchgate.net
Spherical plots of spherical harmonics for l = 0, 1, 2, 3 and m = −l Spherical Node Mean If $r(r_1) = 0$, there exists a radial node. The main difference between radial. Nodes are regions in atoms where no electron can ever be found. The radial node is a sphere with radius $r_1$. Radial nodes are nodes inside the orbital lobes as far as i can understand. A nodal plane is also called an angular node, which is. Spherical Node Mean.
From byjus.com
What is the difference between Angular node and Spherical node Spherical Node Mean A nodal surface is also called a radial node, which is a hollow spherical region in which electrons cannot be. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. The radial node is a sphere with radius $r_1$. The minima correspond to spherical nodes (regions of zero electron probability),. Spherical Node Mean.
From www.meritnation.com
Does spherical node mean the same as angular node Do we use the formula Spherical Node Mean Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. Radial nodes are nodes inside the orbital lobes as far as i can understand. A radial node is a sphere (rather than an angular node which is a flat plane) that occurs when the radial wavefunction for an atomic orbital. Spherical Node Mean.
From quizlet.com
Two types of nodes occur in atomic orbitals spherical surfa Quizlet Spherical Node Mean Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. Nodes are regions in atoms where no electron can ever be found. Therefore the terms radial node and spherical node are the same. The minima correspond to spherical nodes (regions of zero electron probability), which alternate with spherical regions of. Spherical Node Mean.
From chem.libretexts.org
s Atomic Orbitals Chemistry LibreTexts Spherical Node Mean The main difference between radial. A nodal plane is also called an angular node, which is either a plane. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. The minima correspond to spherical nodes (regions of zero electron probability), which alternate with spherical regions of nonzero. A nodal surface. Spherical Node Mean.
From chem.libretexts.org
0.1.1 Review of Orbital Shapes Chemistry LibreTexts Spherical Node Mean Nodes are regions in atoms where no electron can ever be found. The radial node is a sphere with radius $r_1$. The main difference between radial. The minima correspond to spherical nodes (regions of zero electron probability), which alternate with spherical regions of nonzero. A radial node is a sphere (rather than an angular node which is a flat plane). Spherical Node Mean.
From www.youtube.com
Spherical Geometry Deriving The Formula For The Area Of A Spherical Spherical Node Mean A radial node is a sphere (rather than an angular node which is a flat plane) that occurs when the radial wavefunction for an atomic orbital is equal to zero or. There are two types of nodes as radial nodes and angular nodes. A nodal surface is also called a radial node, which is a hollow spherical region in which. Spherical Node Mean.
From www.researchgate.net
Plot of the concentric spherical Areas of AntiNode of the Spherical Node Mean Nodes are regions in atoms where no electron can ever be found. Therefore the terms radial node and spherical node are the same. If $r(r_1) = 0$, there exists a radial node. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like for p orbitals. There are two types of nodes as radial. Spherical Node Mean.
From ms.copernicus.org
MS Dimensional synthesis of a spherical linkage crank slider Spherical Node Mean The minima correspond to spherical nodes (regions of zero electron probability), which alternate with spherical regions of nonzero. Radial nodes are nodes inside the orbital lobes as far as i can understand. Therefore the terms radial node and spherical node are the same. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like. Spherical Node Mean.
From www.researchgate.net
Quadrature nodes on the sphere for GaussLegendre rule (GL), symmetric Spherical Node Mean The radial node is a sphere with radius $r_1$. There are two types of nodes as radial nodes and angular nodes. If $r(r_1) = 0$, there exists a radial node. Therefore the terms radial node and spherical node are the same. The main difference between radial. A nodal plane is also called an angular node, which is either a plane.. Spherical Node Mean.
From www.youtube.com
2.28Node, spherical node/radial node and nodal plane/angular node Spherical Node Mean A radial node is a sphere (rather than an angular node which is a flat plane) that occurs when the radial wavefunction for an atomic orbital is equal to zero or. Nodes are regions in atoms where no electron can ever be found. Analogically, a constant angle of angular nodes leads to a 3d plane intersecting the coordinate origin, like. Spherical Node Mean.
From www.researchgate.net
Spherical particles distributed in the same node or antinode plane of Spherical Node Mean If $r(r_1) = 0$, there exists a radial node. The radial node is a sphere with radius $r_1$. A nodal plane is also called an angular node, which is either a plane. Radial nodes are nodes inside the orbital lobes as far as i can understand. The main difference between radial. Therefore the terms radial node and spherical node are. Spherical Node Mean.
From www.chegg.com
Solved a) Two types of nodes occur in atomic orbitals Spherical Node Mean Nodes are regions in atoms where no electron can ever be found. A nodal surface is also called a radial node, which is a hollow spherical region in which electrons cannot be. The main difference between radial. Therefore the terms radial node and spherical node are the same. There are two types of nodes as radial nodes and angular nodes.. Spherical Node Mean.
From www.numerade.com
SOLVED Boundary Surface Dot Picture This is the 3 dx y orbital. How Spherical Node Mean A nodal surface is also called a radial node, which is a hollow spherical region in which electrons cannot be. There are two types of nodes as radial nodes and angular nodes. A nodal plane is also called an angular node, which is either a plane. Nodes are regions in atoms where no electron can ever be found. Analogically, a. Spherical Node Mean.
From www.slideserve.com
PPT For an animation of spherical coordinates visit PowerPoint Spherical Node Mean A radial node is a sphere (rather than an angular node which is a flat plane) that occurs when the radial wavefunction for an atomic orbital is equal to zero or. The main difference between radial. If $r(r_1) = 0$, there exists a radial node. The radial node is a sphere with radius $r_1$. Therefore the terms radial node and. Spherical Node Mean.