Urea Protein Denaturation Temperature . The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. A criterion for protein stability is the temperature difference between heat and cold denaturation. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and.
from www3.nd.edu
A criterion for protein stability is the temperature difference between heat and cold denaturation. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and.
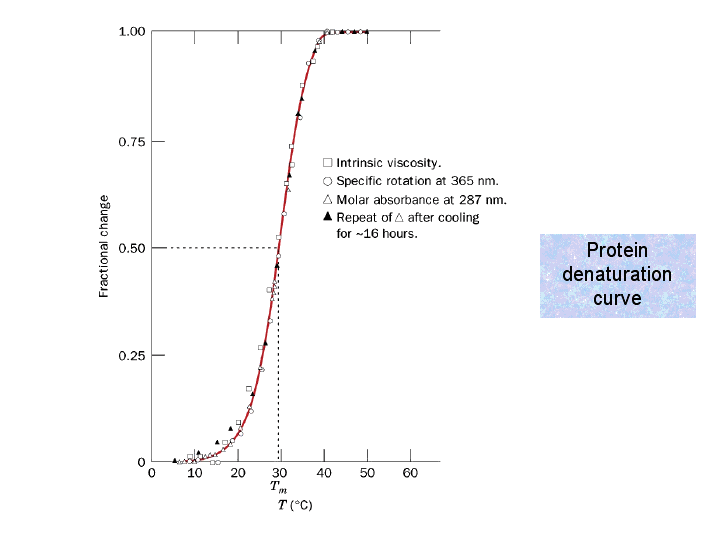
Protein denaturation curve
Urea Protein Denaturation Temperature Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. A criterion for protein stability is the temperature difference between heat and cold denaturation. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and.
From www.frontiersin.org
Frontiers Protein stability [determination] problems Urea Protein Denaturation Temperature The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. A criterion for protein stability is the temperature difference between heat and cold denaturation. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction. Urea Protein Denaturation Temperature.
From www3.nd.edu
Protein denaturation curve Urea Protein Denaturation Temperature Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. A criterion for protein stability is the temperature difference between heat and cold denaturation. Thermal shift assay (tsa) with altered temperature has been the most widely used. Urea Protein Denaturation Temperature.
From www.researchgate.net
Simulations on the evolution of protein denaturation temperature under Urea Protein Denaturation Temperature A criterion for protein stability is the temperature difference between heat and cold denaturation. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction. Urea Protein Denaturation Temperature.
From www.keaipublishing.com
Researchers shine new light on how urea denatures protein KeAi Publishing Urea Protein Denaturation Temperature Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. A criterion for protein stability is the temperature difference between heat and cold denaturation. The stability of globular proteins is an important factor in determining their usefulness in. Urea Protein Denaturation Temperature.
From www.researchgate.net
Normalized ureainduced denaturation curves of WT ycytc and deletants Urea Protein Denaturation Temperature Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. A criterion for protein stability is the temperature difference between heat and cold denaturation. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction. Urea Protein Denaturation Temperature.
From www.pnas.org
Counteraction of ureainduced protein denaturation by trimethylamine N Urea Protein Denaturation Temperature Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. A criterion for protein stability is the temperature difference between heat and cold denaturation. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Thermal shift assay (tsa) with altered temperature has been the most widely used. Urea Protein Denaturation Temperature.
From www.pnas.org
The molecular basis for the chemical denaturation of proteins by urea Urea Protein Denaturation Temperature A criterion for protein stability is the temperature difference between heat and cold denaturation. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. The stability of globular proteins is an important factor in determining their usefulness in. Urea Protein Denaturation Temperature.
From pubs.acs.org
Equilibrium Study of Protein Denaturation by Urea Journal of the Urea Protein Denaturation Temperature Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. A criterion for protein stability is the temperature difference between heat and cold denaturation. The stability of globular proteins is an important factor in determining their usefulness in. Urea Protein Denaturation Temperature.
From www.researchgate.net
(PDF) Native protein denaturation using urea Urea Protein Denaturation Temperature Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. A criterion for protein stability is the temperature difference between. Urea Protein Denaturation Temperature.
From www.researchgate.net
(A) Urea denaturation curves allow to estimate the effect of the pH on Urea Protein Denaturation Temperature A criterion for protein stability is the temperature difference between heat and cold denaturation. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Thermal shift assay (tsa) with altered temperature has been the most widely used. Urea Protein Denaturation Temperature.
From www.slideserve.com
PPT Protein Structure PowerPoint Presentation, free download ID1922177 Urea Protein Denaturation Temperature Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. A criterion for protein stability is the temperature difference between heat and cold denaturation. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Thermal shift assay (tsa) with altered temperature has been the most widely used. Urea Protein Denaturation Temperature.
From www.researchgate.net
Thermal denaturation profile of Myoglobin protein after treatment with Urea Protein Denaturation Temperature Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. A criterion for protein stability is the temperature difference between heat and cold denaturation. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction. Urea Protein Denaturation Temperature.
From www.differencebetween.com
Difference Between Protein Denaturation and Hydrolysis Compare the Urea Protein Denaturation Temperature Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. A criterion for protein stability is the temperature difference between heat and cold denaturation. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Thermal shift assay (tsa) with altered temperature has been the most widely used. Urea Protein Denaturation Temperature.
From www.pnas.org
Urea denaturation by stronger dispersion interactions with proteins Urea Protein Denaturation Temperature The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. A criterion for protein stability is the temperature difference between heat and cold denaturation. Thermal shift assay (tsa) with altered temperature has been the most widely used. Urea Protein Denaturation Temperature.
From byjus.com
During the denaturation of proteins, which of these structures will Urea Protein Denaturation Temperature A criterion for protein stability is the temperature difference between heat and cold denaturation. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. The stability of globular proteins is an important factor in determining their usefulness in. Urea Protein Denaturation Temperature.
From www.researchgate.net
DSC thermogram for protein denaturation. Peaks 1, 2, and 3 represent Urea Protein Denaturation Temperature A criterion for protein stability is the temperature difference between heat and cold denaturation. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. The stability of globular proteins is an important factor in determining their usefulness in. Urea Protein Denaturation Temperature.
From pubs.rsc.org
The shift in urea orientation at protein surfaces at low pH is Urea Protein Denaturation Temperature Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. A criterion for protein stability is the temperature difference between. Urea Protein Denaturation Temperature.
From chem.libretexts.org
4.5 Laboratory Determination of ΔG° of Protein Unfolding Chemistry Urea Protein Denaturation Temperature The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. A criterion for protein stability is the temperature difference between. Urea Protein Denaturation Temperature.
From www.researchgate.net
Thermal denaturation curves and Tm identification. (A) A typical Urea Protein Denaturation Temperature A criterion for protein stability is the temperature difference between heat and cold denaturation. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. The stability of globular proteins is an important factor in determining their usefulness in. Urea Protein Denaturation Temperature.
From en.wikipedia.org
Denaturation (biochemistry) Wikipedia Urea Protein Denaturation Temperature Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. A criterion for protein stability is the temperature difference between. Urea Protein Denaturation Temperature.
From www.researchgate.net
Schematic illustration of the urea induced CP denaturation via indirect Urea Protein Denaturation Temperature Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. A criterion for protein stability is the temperature difference between heat and cold denaturation. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. The stability of globular proteins is an important factor in determining their usefulness in. Urea Protein Denaturation Temperature.
From nienkebrayan.blogspot.com
11+ Body Melt System NienkeBrayan Urea Protein Denaturation Temperature Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. A criterion for protein stability is the temperature difference between heat and cold denaturation. The stability of globular proteins is an important factor in determining their usefulness in. Urea Protein Denaturation Temperature.
From byjus.com
Denaturation Of Proteins By Urea Enzymes Of Denatured Ph Value Urea Protein Denaturation Temperature A criterion for protein stability is the temperature difference between heat and cold denaturation. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. Thermal shift assay (tsa) with altered temperature has been the most widely used. Urea Protein Denaturation Temperature.
From www.researchgate.net
Figure S1. A) Denaturing PAGE (20 , 7M urea) analysis of... Urea Protein Denaturation Temperature The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. A criterion for protein stability is the temperature difference between heat and cold denaturation. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction. Urea Protein Denaturation Temperature.
From www.researchgate.net
Stability analysis of NRN1L.h.variants. Protein denaturation was Urea Protein Denaturation Temperature Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. A criterion for protein stability is the temperature difference between. Urea Protein Denaturation Temperature.
From www.researchgate.net
Simulations on the evolution of protein denaturation temperature under Urea Protein Denaturation Temperature The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. A criterion for protein stability is the temperature difference between heat and cold denaturation. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. Thermal shift assay (tsa) with altered temperature has been the most widely used. Urea Protein Denaturation Temperature.
From www.researchgate.net
Normalized equilibrium urea denaturation curves measured by tyrosine Urea Protein Denaturation Temperature Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. A criterion for protein stability is the temperature difference between. Urea Protein Denaturation Temperature.
From slideplayer.com
BIOCHEMISTRY The chemistry of the carbon atom Versatility of the carbon Urea Protein Denaturation Temperature A criterion for protein stability is the temperature difference between heat and cold denaturation. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. The stability of globular proteins is an important factor in determining their usefulness in. Urea Protein Denaturation Temperature.
From www.slideserve.com
PPT Protein Structure PowerPoint Presentation, free download ID1922177 Urea Protein Denaturation Temperature Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. A criterion for protein stability is the temperature difference between. Urea Protein Denaturation Temperature.
From www.pnas.org
The molecular basis for the chemical denaturation of proteins by urea Urea Protein Denaturation Temperature Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. A criterion for protein stability is the temperature difference between heat and cold denaturation. The stability of globular proteins is an important factor in determining their usefulness in. Urea Protein Denaturation Temperature.
From www.researchgate.net
(a) Schematic illustration of the urea?induced CP denaturation via Urea Protein Denaturation Temperature Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. A criterion for protein stability is the temperature difference between heat and cold denaturation. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. The stability of globular proteins is an important factor in determining their usefulness in. Urea Protein Denaturation Temperature.
From www.slideserve.com
PPT Organization of DNA PowerPoint Presentation, free download ID Urea Protein Denaturation Temperature Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. A criterion for protein stability is the temperature difference between heat and cold denaturation. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. The stability of globular proteins is an important factor in determining their usefulness in. Urea Protein Denaturation Temperature.
From chemistrytalk.org
Denaturation of Proteins What is it? ChemTalk Urea Protein Denaturation Temperature A criterion for protein stability is the temperature difference between heat and cold denaturation. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. The stability of globular proteins is an important factor in determining their usefulness in. Urea Protein Denaturation Temperature.
From www.slideserve.com
PPT Denaturation of proteins by heat and guanidine hydrochloride Urea Protein Denaturation Temperature Thermal shift assay (tsa) with altered temperature has been the most widely used method for monitoring protein. The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. A criterion for protein stability is the temperature difference between heat and cold denaturation. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction. Urea Protein Denaturation Temperature.
From www.researchgate.net
Ureainduced denaturation of BL was monitored by changes in secondary Urea Protein Denaturation Temperature The stability of globular proteins is an important factor in determining their usefulness in basic research and medicine. Solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic residues, and. A criterion for protein stability is the temperature difference between heat and cold denaturation. Thermal shift assay (tsa) with altered temperature has been the most widely used. Urea Protein Denaturation Temperature.