Titration Curve Guide . Here are reduced versions of. Ph titration curve for a strong acid + weak alkali. The way you normally carry out a titration involves adding the acid to the alkali. A summary of the important curves. What is the difference between endpoint and equivalence point titration? The ph titration curve for strong acid added to a weak. What is a titration curve? A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. How to achieve accurate titration results?. Both equivalence points are visible. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together. Titration is a procedure which a skilled practitioner can perform with a high degree of both precision and accuracy. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong.
from general.chemistrysteps.com
The ph titration curve for strong acid added to a weak. Ph titration curve for a strong acid + weak alkali. Both equivalence points are visible. Titration is a procedure which a skilled practitioner can perform with a high degree of both precision and accuracy. What is a titration curve? Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together. The way you normally carry out a titration involves adding the acid to the alkali. How to achieve accurate titration results?. What is the difference between endpoint and equivalence point titration? A titration curve is a graphical representation of the ph of a solution during a titration.
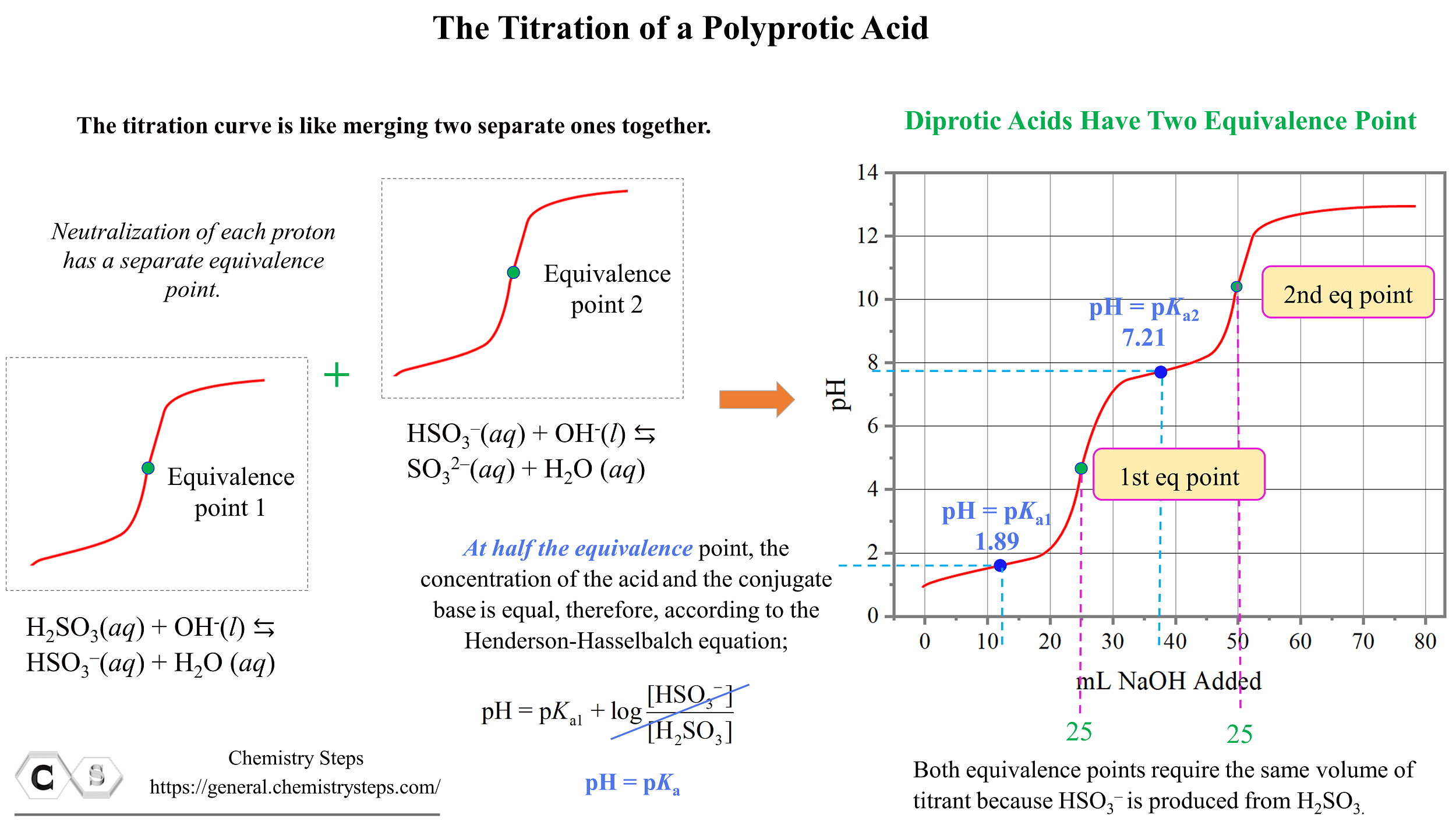
Titration of a Polyprotic Acids Chemistry Steps
Titration Curve Guide The way you normally carry out a titration involves adding the acid to the alkali. How to achieve accurate titration results?. Titration is a procedure which a skilled practitioner can perform with a high degree of both precision and accuracy. The ph titration curve for strong acid added to a weak. What is a titration curve? A titration curve is a graphical representation of the ph of a solution during a titration. A summary of the important curves. Both equivalence points are visible. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together. Here are reduced versions of. Ph titration curve for a strong acid + weak alkali. What is the difference between endpoint and equivalence point titration? A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The figure below shows two different examples of a strong. The way you normally carry out a titration involves adding the acid to the alkali.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Curve Guide What is the difference between endpoint and equivalence point titration? A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Both equivalence points are visible. The figure below shows two different examples of a strong. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential. Titration Curve Guide.
From www.numerade.com
SOLVED Part D Interpreting Titration Curves Using the graphs shown Titration Curve Guide The ph titration curve for strong acid added to a weak. A titration curve is a graphical representation of the ph of a solution during a titration. The way you normally carry out a titration involves adding the acid to the alkali. Here are reduced versions of. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be. Titration Curve Guide.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Guide A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. How to achieve accurate titration results?. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together. Ph titration curve for a strong acid + weak alkali. A summary of. Titration Curve Guide.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Guide How to achieve accurate titration results?. The figure below shows two different examples of a strong. Ph titration curve for a strong acid + weak alkali. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A summary of the important curves. Both equivalence points are visible. A titration curve is a graphical. Titration Curve Guide.
From www.chegg.com
Solved 5. The titration curve below is two amino acids. Titration Curve Guide A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. How to achieve accurate titration results?. The way you normally carry out a titration involves adding the acid to the alkali. What is the difference between endpoint and equivalence point titration? Both equivalence points are visible. The figure below shows two different examples. Titration Curve Guide.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Curve Guide Ph titration curve for a strong acid + weak alkali. How to achieve accurate titration results?. What is the difference between endpoint and equivalence point titration? Titration is a procedure which a skilled practitioner can perform with a high degree of both precision and accuracy. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base,. Titration Curve Guide.
From www.albert.io
[HF] and [F^] Comparison from a Titration Curve AP® Chemistry Titration Curve Guide Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together. Both equivalence points are visible. How to achieve accurate titration results?. A summary of the important curves. Titration is a procedure which a skilled practitioner can perform with a high degree of both precision and accuracy.. Titration Curve Guide.
From webmis.highland.cc.il.us
AcidBase Titrations Titration Curve Guide A titration curve is a graphical representation of the ph of a solution during a titration. What is the difference between endpoint and equivalence point titration? The figure below shows two different examples of a strong. How to achieve accurate titration results?. Ph titration curve for a strong acid + weak alkali. The ph titration curve for strong acid added. Titration Curve Guide.
From www.vrogue.co
Acid Base Titration Curves Youtube vrogue.co Titration Curve Guide Here are reduced versions of. How to achieve accurate titration results?. A titration curve is a graphical representation of the ph of a solution during a titration. The way you normally carry out a titration involves adding the acid to the alkali. Both equivalence points are visible. What is the difference between endpoint and equivalence point titration? The figure below. Titration Curve Guide.
From www.researchgate.net
Theoretical titration curves. The calculated curves are shown in red Titration Curve Guide Both equivalence points are visible. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A titration curve is a graphical representation of the ph of a solution during a titration. The way you normally carry out a titration involves adding the acid to the alkali. The ph titration curve for strong acid. Titration Curve Guide.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Guide A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The ph titration curve for strong acid added to a weak. Both equivalence points are visible. A titration curve is a graphical representation of the ph of a solution during a titration. The way you normally carry out a titration involves adding the. Titration Curve Guide.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5570905 Titration Curve Guide A titration curve is a graphical representation of the ph of a solution during a titration. Both equivalence points are visible. What is a titration curve? The way you normally carry out a titration involves adding the acid to the alkali. The figure below shows two different examples of a strong. The ph titration curve for strong acid added to. Titration Curve Guide.
From studyfinder.org
The Ultimate Guide to Understanding and Interpreting Titration Curves Titration Curve Guide Ph titration curve for a strong acid + weak alkali. Both equivalence points are visible. What is the difference between endpoint and equivalence point titration? What is a titration curve? How to achieve accurate titration results?. The ph titration curve for strong acid added to a weak. The way you normally carry out a titration involves adding the acid to. Titration Curve Guide.
From chem.libretexts.org
11.3 Reaction Stoichiometry in Solutions AcidBase Titrations Titration Curve Guide Here are reduced versions of. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Titration is a procedure which a skilled practitioner can perform with a high degree of both precision and accuracy. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below. Titration Curve Guide.
From www.pearson.com
The graphs labeled (a) and (b) show the titration curves for two Titration Curve Guide How to achieve accurate titration results?. What is the difference between endpoint and equivalence point titration? Ph titration curve for a strong acid + weak alkali. The figure below shows two different examples of a strong. The way you normally carry out a titration involves adding the acid to the alkali. A summary of the important curves. What is a. Titration Curve Guide.
From www.afterskool.com.sg
HL/ H2 Chemistry 101 Titration Curves Summary Guide — AfterSkool Titration Curve Guide Titration is a procedure which a skilled practitioner can perform with a high degree of both precision and accuracy. What is the difference between endpoint and equivalence point titration? Ph titration curve for a strong acid + weak alkali. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two. Titration Curve Guide.
From www.writework.com
Titration of amino acids WriteWork Titration Curve Guide A titration curve is a graphical representation of the ph of a solution during a titration. Ph titration curve for a strong acid + weak alkali. What is a titration curve? Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together. A typical titration curve of. Titration Curve Guide.
From www.researchgate.net
Potentiometric titration curve of blank and (0.5g, 1g and 1.5g) of red Titration Curve Guide The ph titration curve for strong acid added to a weak. A summary of the important curves. Both equivalence points are visible. Here are reduced versions of. The way you normally carry out a titration involves adding the acid to the alkali. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. What. Titration Curve Guide.
From www.chegg.com
Solved Titration curve 14 12 10 6 4 0 0 10 20 30 40 50 60 70 Titration Curve Guide The figure below shows two different examples of a strong. What is a titration curve? A summary of the important curves. Ph titration curve for a strong acid + weak alkali. How to achieve accurate titration results?. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. What is the difference between endpoint. Titration Curve Guide.
From pressbooks.bccampus.ca
14.7 AcidBase Titrations Chemistry 2e for Chem 120 (Chemistry for Titration Curve Guide Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. How to achieve accurate titration results?. Both equivalence points are visible. What is the difference between endpoint and equivalence. Titration Curve Guide.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Guide Titration is a procedure which a skilled practitioner can perform with a high degree of both precision and accuracy. Here are reduced versions of. What is a titration curve? Ph titration curve for a strong acid + weak alkali. What is the difference between endpoint and equivalence point titration? Using a ph/millivoltmeter, a titration curve e or ph = f(volume). Titration Curve Guide.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Guide The ph titration curve for strong acid added to a weak. What is the difference between endpoint and equivalence point titration? The way you normally carry out a titration involves adding the acid to the alkali. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. How to achieve accurate titration results?. A. Titration Curve Guide.
From www.flinnsci.ca
Classic Titration pH Curves and an Unknown—ChemTopic™ Lab Activity Titration Curve Guide Titration is a procedure which a skilled practitioner can perform with a high degree of both precision and accuracy. How to achieve accurate titration results?. Ph titration curve for a strong acid + weak alkali. Both equivalence points are visible. The ph titration curve for strong acid added to a weak. Here are reduced versions of. The way you normally. Titration Curve Guide.
From www.jove.com
AcidBase Titration Curves JoVE Titration Curve Guide Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Titration is a procedure which a skilled practitioner can perform with a high degree of both precision and accuracy.. Titration Curve Guide.
From ambitiousmares.blogspot.com
30 Label The Important Points Of The Titration Curve Below. Labels Titration Curve Guide The way you normally carry out a titration involves adding the acid to the alkali. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Both equivalence points are visible. Here are reduced versions of. Ph titration curve for a strong acid + weak alkali. The ph titration curve for strong acid added. Titration Curve Guide.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Curve Guide The figure below shows two different examples of a strong. Titration is a procedure which a skilled practitioner can perform with a high degree of both precision and accuracy. Both equivalence points are visible. The ph titration curve for strong acid added to a weak. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base,. Titration Curve Guide.
From chem.libretexts.org
11.3 Reaction Stoichiometry in Solutions AcidBase Titrations Titration Curve Guide A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The way you normally carry out a titration involves adding the acid to the alkali. Here are reduced versions of. A summary of the important curves. Both equivalence points are visible. A titration curve is a graphical representation of the ph of a. Titration Curve Guide.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Curve Guide The figure below shows two different examples of a strong. The way you normally carry out a titration involves adding the acid to the alkali. The ph titration curve for strong acid added to a weak. Here are reduced versions of. What is a titration curve? A summary of the important curves. A typical titration curve of a diprotic acid,. Titration Curve Guide.
From www.bartleby.com
The graph shows the titration curves for two monoprotic Titration Curve Guide Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together. The ph titration curve for strong acid added to a weak. Here are reduced versions of. What is the difference between endpoint and equivalence point titration? A summary of the important curves. The way you normally. Titration Curve Guide.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Guide A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A summary of the important curves. Ph titration curve for a strong acid + weak alkali. How to achieve accurate titration results?. Titration is a procedure which a skilled practitioner can perform with a high degree of both precision and accuracy. Here are. Titration Curve Guide.
From www.numerade.com
SOLVED Refer to the acidbase titration curve shown below. This figure Titration Curve Guide The ph titration curve for strong acid added to a weak. The way you normally carry out a titration involves adding the acid to the alkali. What is the difference between endpoint and equivalence point titration? Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together.. Titration Curve Guide.
From www.animalia-life.club
Titration Curve Amino Acid Titration Curve Guide Ph titration curve for a strong acid + weak alkali. What is the difference between endpoint and equivalence point titration? A titration curve is a graphical representation of the ph of a solution during a titration. The way you normally carry out a titration involves adding the acid to the alkali. A summary of the important curves. Using a ph/millivoltmeter,. Titration Curve Guide.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Guide Here are reduced versions of. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Both equivalence points are visible. A titration curve is a graphical representation of the ph of a solution during a titration. Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the. Titration Curve Guide.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.7.12 pH Titration Curves翰林国际教育 Titration Curve Guide Here are reduced versions of. How to achieve accurate titration results?. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. What is the difference between endpoint and equivalence point titration? Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator. Titration Curve Guide.
From solvedlib.com
The graph below shows the titration curves for two mo… SolvedLib Titration Curve Guide Using a ph/millivoltmeter, a titration curve e or ph = f(volume) can be plotted by following the potential e of an indicator electrode (together. Both equivalence points are visible. What is a titration curve? The way you normally carry out a titration involves adding the acid to the alkali. A typical titration curve of a diprotic acid, oxalic acid, titrated. Titration Curve Guide.