Electrolysis Of Copper Ii Nitrate Solution . In this practical, students get the chance. Copper is deposited at the cathode as you would expect, but. a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. Copper(ii) ions and hydrogen ions arrive. the electrolysis of copper(ii) sulfate solution using inert electrodes. electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. In copper electrolysis, when a. try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. revision notes on electrolysis of aqueous solutions for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. This page looks in detail at the. Copper is below hydrogen in the. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the.
from www.alamy.com
try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. Copper is deposited at the cathode as you would expect, but. Copper(ii) ions and hydrogen ions arrive. electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. In copper electrolysis, when a. Copper is below hydrogen in the. electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. This page looks in detail at the. a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. In this practical, students get the chance.
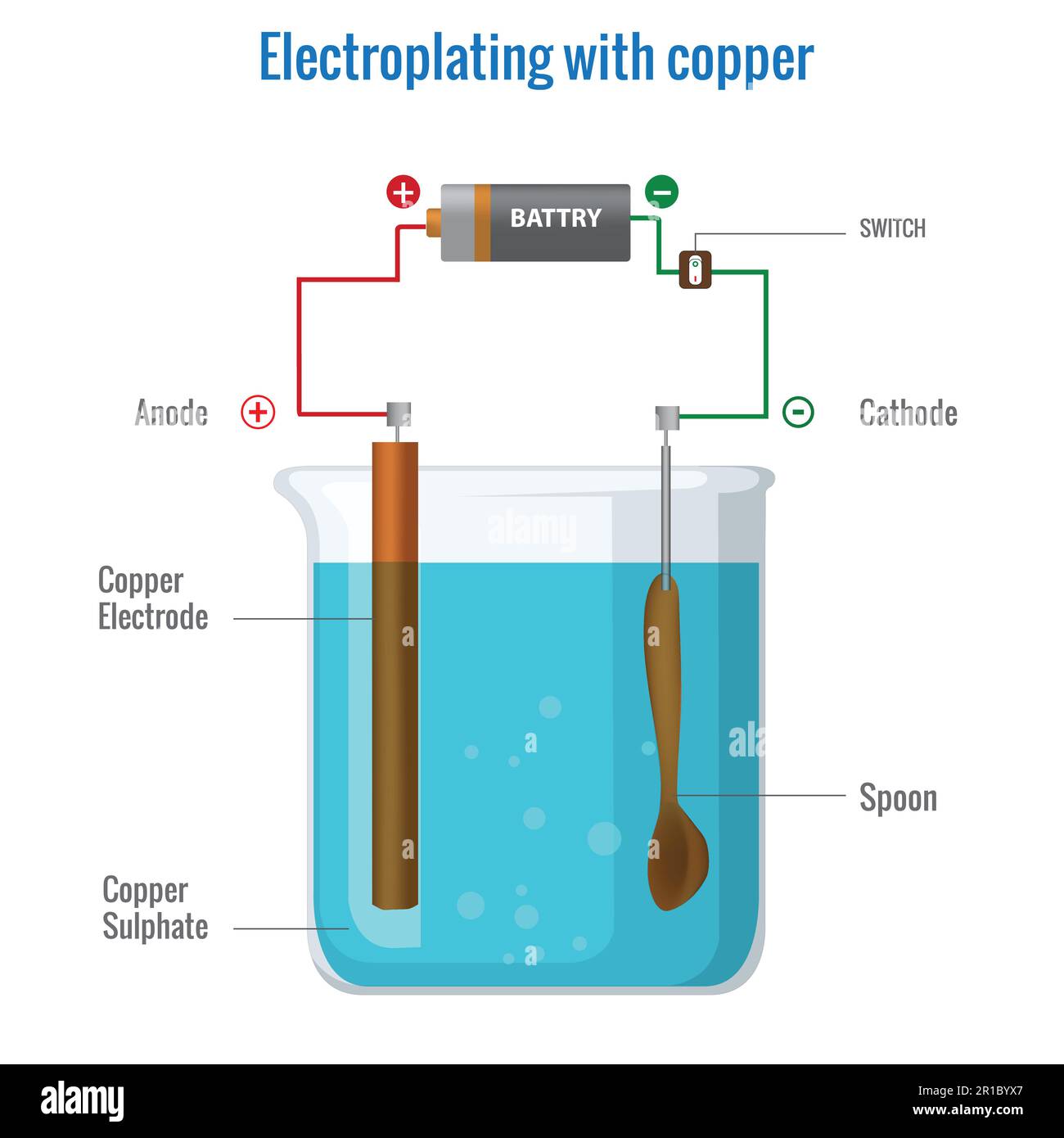
Electroplating with copper using copper sulfate electrolyte. Electrolysis of copper(II) sulfate
Electrolysis Of Copper Ii Nitrate Solution Copper(ii) ions and hydrogen ions arrive. This page looks in detail at the. electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. Copper(ii) ions and hydrogen ions arrive. a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. In copper electrolysis, when a. In this practical, students get the chance. Copper is deposited at the cathode as you would expect, but. revision notes on electrolysis of aqueous solutions for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. the electrolysis of copper(ii) sulfate solution using inert electrodes. Copper is below hydrogen in the.
From www.nagwa.com
Question Video Writing the Equation for the Reaction at the Anode during the Electrolysis of Electrolysis Of Copper Ii Nitrate Solution Copper is below hydrogen in the. In copper electrolysis, when a. This page looks in detail at the. try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then. Electrolysis Of Copper Ii Nitrate Solution.
From www.youtube.com
Chemistry and Double Investigation Copper Electrolysis 2016 YouTube Electrolysis Of Copper Ii Nitrate Solution This page looks in detail at the. revision notes on electrolysis of aqueous solutions for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. In copper electrolysis, when a. In this practical, students get the chance. the electrolysis of copper(ii) sulfate solution using inert electrodes. try this class experiment to carry out. Electrolysis Of Copper Ii Nitrate Solution.
From www.quanswer.com
What happens to the colour of copper 2 nitrate during electrolysis? Quanswer Electrolysis Of Copper Ii Nitrate Solution electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. In this practical, students get the chance. Copper is deposited at the cathode as you would expect, but. Copper(ii) ions and hydrogen ions arrive. try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. In. Electrolysis Of Copper Ii Nitrate Solution.
From www.linstitute.net
IB DP Chemistry HL复习笔记19.1.3 Electrolysis of Aqueous Solutions翰林国际教育 Electrolysis Of Copper Ii Nitrate Solution try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. In this practical, students get the chance. This page looks in. Electrolysis Of Copper Ii Nitrate Solution.
From stock.adobe.com
Electrolysis of copper. Electrolytic reduction of copper from solution. electrolysis in the Electrolysis Of Copper Ii Nitrate Solution the electrolysis of copper(ii) sulfate solution using inert electrodes. electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. revision notes on electrolysis of aqueous solutions for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Copper(ii) ions and hydrogen ions arrive. a similar change. Electrolysis Of Copper Ii Nitrate Solution.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte. Electrolysis of copper(II) sulfate Electrolysis Of Copper Ii Nitrate Solution Copper(ii) ions and hydrogen ions arrive. Copper is deposited at the cathode as you would expect, but. the electrolysis of copper(ii) sulfate solution using inert electrodes. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. electrolysis of a copper(ii) nitrate solution produces. Electrolysis Of Copper Ii Nitrate Solution.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Electrolysis Of Copper Ii Nitrate Solution with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. Copper(ii) ions and hydrogen ions arrive. This page looks in detail at the. In copper electrolysis, when a. In this practical, students get the chance. a similar change happens if you electrolyse copper(ii) sulphate. Electrolysis Of Copper Ii Nitrate Solution.
From schematicodtautobuses5n.z4.web.core.windows.net
What Forms At The Cathode During Electrolysis Electrolysis Of Copper Ii Nitrate Solution the electrolysis of copper(ii) sulfate solution using inert electrodes. electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. revision notes on electrolysis of aqueous solutions for the cie igcse chemistry syllabus, written by the chemistry experts at. Electrolysis Of Copper Ii Nitrate Solution.
From library.fiveable.me
Electrode and Cell Potentials Intro to Chemistry Study Guide 2024 Fiveable Electrolysis Of Copper Ii Nitrate Solution electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. Copper(ii) ions and hydrogen ions arrive. try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. Copper is below. Electrolysis Of Copper Ii Nitrate Solution.
From www.youtube.com
Electrolysis of impure copper YouTube Electrolysis Of Copper Ii Nitrate Solution a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. This page looks in detail at the. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. In copper electrolysis, when a. Copper is below hydrogen in the. In this practical,. Electrolysis Of Copper Ii Nitrate Solution.
From www.youtube.com
Electrolysis Of Copper(ii) Sulphate Using Copper Electrodes YouTube Electrolysis Of Copper Ii Nitrate Solution This page looks in detail at the. try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. the electrolysis of copper(ii) sulfate solution using inert electrodes. Copper is below hydrogen in the. electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. electrolysis. Electrolysis Of Copper Ii Nitrate Solution.
From chem2u.blogspot.com
chem2U Electrolysis of Copper(II) Sulphate Electrolysis Of Copper Ii Nitrate Solution In copper electrolysis, when a. In this practical, students get the chance. This page looks in detail at the. try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. Copper is below hydrogen in the. the electrolysis of copper(ii) sulfate solution using inert electrodes. with ph near neutral (ph =. Electrolysis Of Copper Ii Nitrate Solution.
From www.youtube.com
Electrolysis of Copper(ii) Sulphate Using Graphite YouTube Electrolysis Of Copper Ii Nitrate Solution Copper is below hydrogen in the. Copper(ii) ions and hydrogen ions arrive. Copper is deposited at the cathode as you would expect, but. electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. electrolysis of a copper(ii) nitrate solution. Electrolysis Of Copper Ii Nitrate Solution.
From www.flinnsci.com
Flinn Chemicals, Copper(II) Nitrate Solution Electrolysis Of Copper Ii Nitrate Solution Copper is deposited at the cathode as you would expect, but. try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. In copper electrolysis, when a. the electrolysis of copper(ii) sulfate solution using inert electrodes.. Electrolysis Of Copper Ii Nitrate Solution.
From www.youtube.com
AQA Required Practical The electrolysis of copper (II) chloride. YouTube Electrolysis Of Copper Ii Nitrate Solution Copper is deposited at the cathode as you would expect, but. the electrolysis of copper(ii) sulfate solution using inert electrodes. In copper electrolysis, when a. revision notes on electrolysis of aqueous solutions for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. try this class experiment to carry out the electrolysis of. Electrolysis Of Copper Ii Nitrate Solution.
From socratic.org
What happens on the cathode during the electrolysis of a copper(II) sulfate solution? Socratic Electrolysis Of Copper Ii Nitrate Solution Copper is deposited at the cathode as you would expect, but. the electrolysis of copper(ii) sulfate solution using inert electrodes. try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to. Electrolysis Of Copper Ii Nitrate Solution.
From studymind.co.uk
Electrolysis of Aqueous Solutions (GCSE Chemistry) Study Mind Electrolysis Of Copper Ii Nitrate Solution Copper is below hydrogen in the. electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. In this practical, students get the chance. revision notes on electrolysis of aqueous solutions for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. the electrolysis of copper(ii) sulfate solution. Electrolysis Of Copper Ii Nitrate Solution.
From www.vecteezy.com
Electrolysis of copper sulfate solution with impure copper anode and pure copper cathode Electrolysis Of Copper Ii Nitrate Solution Copper is below hydrogen in the. In this practical, students get the chance. Copper is deposited at the cathode as you would expect, but. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. Copper(ii) ions and hydrogen ions arrive. the electrolysis of copper(ii). Electrolysis Of Copper Ii Nitrate Solution.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrolysis Of Copper Ii Nitrate Solution revision notes on electrolysis of aqueous solutions for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. Copper is deposited at the cathode as you would expect, but. electrolysis uses an electrical current to move ions in an electrolyte. Electrolysis Of Copper Ii Nitrate Solution.
From electro-lysis.blogspot.com
Electro electricity lysisbreak down Selective Discharge and the purification of Copper Electrolysis Of Copper Ii Nitrate Solution Copper(ii) ions and hydrogen ions arrive. a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. In copper electrolysis, when a. electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. . Electrolysis Of Copper Ii Nitrate Solution.
From www.chegg.com
Solved 3. For the electrochemical cell based on the reaction Electrolysis Of Copper Ii Nitrate Solution Copper is below hydrogen in the. In copper electrolysis, when a. electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. try this class experiment to carry out the electrolysis of various solutions and investigate the. Electrolysis Of Copper Ii Nitrate Solution.
From ceujucww.blob.core.windows.net
Inert Electrodes In Electrolysis at Isidro Holland blog Electrolysis Of Copper Ii Nitrate Solution electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. Copper is below hydrogen in the. Copper is deposited at the cathode as you would expect, but. a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. In this practical, students get the chance. Copper(ii) ions and hydrogen ions. Electrolysis Of Copper Ii Nitrate Solution.
From ar.inspiredpencil.com
Copper Electrolytic Cell Electrolysis Of Copper Ii Nitrate Solution try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. revision notes on electrolysis of aqueous solutions for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. This page looks in detail. Electrolysis Of Copper Ii Nitrate Solution.
From byjus.com
With the help of a diagram explain the method of refining of copper by electrolysis. Electrolysis Of Copper Ii Nitrate Solution Copper is deposited at the cathode as you would expect, but. try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. revision notes on electrolysis of aqueous solutions for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Copper(ii) ions and hydrogen ions arrive. In copper. Electrolysis Of Copper Ii Nitrate Solution.
From cepwqyvd.blob.core.windows.net
What Is Electrochemical Properties at Elizabeth Watkins blog Electrolysis Of Copper Ii Nitrate Solution a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. This page looks in detail at the. electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. Copper is below hydrogen in the. electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode.. Electrolysis Of Copper Ii Nitrate Solution.
From www.chegg.com
Solved c) FIGURE 3 shows the electrolysis of copper (II) Electrolysis Of Copper Ii Nitrate Solution In copper electrolysis, when a. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. In this practical, students get the chance. a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. This page looks in detail at the. Copper is. Electrolysis Of Copper Ii Nitrate Solution.
From edu.rsc.org
Electrolysis of copper(II) sulfate solution Experiment RSC Education Electrolysis Of Copper Ii Nitrate Solution electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. Copper is deposited at the cathode as you would expect, but. revision notes on electrolysis of aqueous solutions for the cie igcse chemistry syllabus, written by. Electrolysis Of Copper Ii Nitrate Solution.
From www.elevise.co.uk
C4 K) Electrolysis Part 2 AQA Combined Science Trilogy Elevise Electrolysis Of Copper Ii Nitrate Solution electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. In copper electrolysis, when a. This page looks in detail at the. with ph near neutral (ph = 7), the potential of the nitrate reduction half. Electrolysis Of Copper Ii Nitrate Solution.
From www.sciencephoto.com
Copper(II) nitrate solution Stock Image C043/2656 Science Photo Library Electrolysis Of Copper Ii Nitrate Solution In copper electrolysis, when a. Copper(ii) ions and hydrogen ions arrive. a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. the electrolysis of copper(ii) sulfate solution using inert electrodes. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the.. Electrolysis Of Copper Ii Nitrate Solution.
From saylordotorg.github.io
Electrochemistry Electrolysis Of Copper Ii Nitrate Solution with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. the electrolysis of copper(ii) sulfate solution using inert electrodes. Copper(ii) ions and hydrogen ions arrive. In copper electrolysis, when a. electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper. Electrolysis Of Copper Ii Nitrate Solution.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Electrolysis Of Copper Ii Nitrate Solution a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. In this practical, students get the chance. electrolysis of a copper(ii). Electrolysis Of Copper Ii Nitrate Solution.
From www.carolina.com
Copper (II) Nitrate Solution, 0.1 M, Laboratory Grade, 500 mL Carolina Biological Supply Electrolysis Of Copper Ii Nitrate Solution In copper electrolysis, when a. This page looks in detail at the. Copper(ii) ions and hydrogen ions arrive. try this class experiment to carry out the electrolysis of various solutions and investigate the products formed. electrolysis uses an electrical current to move ions in an electrolyte solution between two electrodes. In this practical, students get the chance. . Electrolysis Of Copper Ii Nitrate Solution.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 Electrolysis Of Copper Ii Nitrate Solution electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. revision notes on electrolysis of aqueous solutions for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. In this practical, students get the chance. Copper is deposited at the cathode as you would expect, but. electrolysis. Electrolysis Of Copper Ii Nitrate Solution.
From fphoto.photoshelter.com
science chemistry electrolysis copper sulfate Fundamental Photographs The Art of Science Electrolysis Of Copper Ii Nitrate Solution This page looks in detail at the. Copper(ii) ions and hydrogen ions arrive. revision notes on electrolysis of aqueous solutions for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Copper is below hydrogen in the. try this class experiment to carry out the electrolysis of various solutions and investigate the products formed.. Electrolysis Of Copper Ii Nitrate Solution.
From www.nagwa.com
Question Video Identifying the Anode Product in the Electrolysis of Nitrate and Sulfate Salts Electrolysis Of Copper Ii Nitrate Solution a similar change happens if you electrolyse copper(ii) sulphate solution using copper electrodes. In copper electrolysis, when a. electrolysis of a copper(ii) nitrate solution produces oxygen at the anode and copper at the cathode. the electrolysis of copper(ii) sulfate solution using inert electrodes. try this class experiment to carry out the electrolysis of various solutions and. Electrolysis Of Copper Ii Nitrate Solution.