Barium Bromide Reacts With Silver Nitrate . This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. Find out what type of reaction occured. Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). The net ionic equation for this. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Balance any equation or reaction using this chemical equation balancer!
from www.chegg.com
Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. Find out what type of reaction occured. Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. Balance any equation or reaction using this chemical equation balancer! This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. The net ionic equation for this. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1).
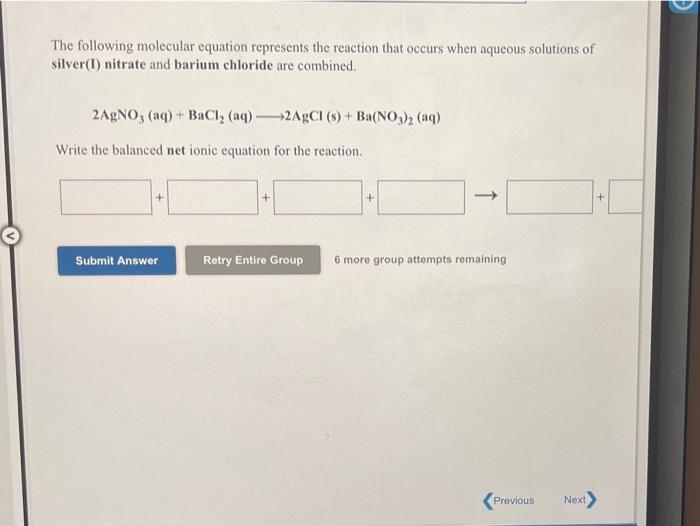
Solved The following molecular equation represents the
Barium Bromide Reacts With Silver Nitrate Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. Find out what type of reaction occured. The net ionic equation for this. Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). Balance any equation or reaction using this chemical equation balancer!
From www.numerade.com
SOLVED Calcium nitrate (aq)+ Sodium phosphate(aq) + Barium chloridelaq Barium Bromide Reacts With Silver Nitrate This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. The net ionic equation for this. Mixing potassium chromate and silver nitrate together. Barium Bromide Reacts With Silver Nitrate.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Barium Bromide Reacts With Silver Nitrate Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. The net ionic equation for this. Mixing potassium chromate. Barium Bromide Reacts With Silver Nitrate.
From mavericksrpatterson.blogspot.com
Chemical Formula of Bromide MavericksrPatterson Barium Bromide Reacts With Silver Nitrate Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Balance any equation or reaction using this chemical equation balancer! This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Find out what. Barium Bromide Reacts With Silver Nitrate.
From www.numerade.com
Silver nitrate reacts with barium chloride to produce solid, silver Barium Bromide Reacts With Silver Nitrate Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. The net ionic equation for this. Balance any equation or reaction using this chemical equation balancer! Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. Mixing. Barium Bromide Reacts With Silver Nitrate.
From www.grainger.com
7758023, F.W. 119.00, Potassium Bromide, Granular, Purified 39G719 Barium Bromide Reacts With Silver Nitrate This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Balance any equation or reaction using this chemical equation balancer! Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). Find out what type of reaction occured. Consider the reaction when aqueous solutions. Barium Bromide Reacts With Silver Nitrate.
From chemcraft.su
Lead(II) bromide, 99.5 pure chemcraft.su Barium Bromide Reacts With Silver Nitrate This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). Balance any equation or reaction using this chemical equation balancer! Two important uses of precipitation reactions are to isolate metals that have been. Barium Bromide Reacts With Silver Nitrate.
From www.numerade.com
SOLVEDPredict whether each of the following reactions will occur in Barium Bromide Reacts With Silver Nitrate Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Balance any equation or reaction using this chemical equation balancer! Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. Mixing potassium chromate and silver nitrate together to initiate a precipitation. Barium Bromide Reacts With Silver Nitrate.
From www.bhphotovideo.com
Photographers' Formulary Sodium Bromide 1 Lb. 101180 1LB B&H Barium Bromide Reacts With Silver Nitrate Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling.. Barium Bromide Reacts With Silver Nitrate.
From www.numerade.com
SOLVED Aqueous solutions of barium chloride and silver nitrate are Barium Bromide Reacts With Silver Nitrate Find out what type of reaction occured. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride. Barium Bromide Reacts With Silver Nitrate.
From askfilo.com
Barium suphare (W) On adding silver nitrate solution to sodium bromide, a.. Barium Bromide Reacts With Silver Nitrate Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. Balance any equation or reaction using this chemical equation balancer! Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores. Barium Bromide Reacts With Silver Nitrate.
From www.sciencecompany.com
Reagent grade Potassium Bromide, 500g for sale. Buy from The Science Barium Bromide Reacts With Silver Nitrate Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. Find out what type of reaction occured. Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). The net ionic equation for. Barium Bromide Reacts With Silver Nitrate.
From www.numerade.com
SOLVED Predict the solubility of the compounds listed below Silver Barium Bromide Reacts With Silver Nitrate Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. Two important uses of precipitation reactions are to isolate metals. Barium Bromide Reacts With Silver Nitrate.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Barium Bromide Reacts With Silver Nitrate Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. This page describes and explains the tests. Barium Bromide Reacts With Silver Nitrate.
From www.chemistrylearner.com
Sodium Bromate Facts, Formula, Properties, Uses, Safety Data Barium Bromide Reacts With Silver Nitrate Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. This page. Barium Bromide Reacts With Silver Nitrate.
From www.numerade.com
SOLVED 1.) The following molecular equation represents the reaction Barium Bromide Reacts With Silver Nitrate The net ionic equation for this. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! This page describes and. Barium Bromide Reacts With Silver Nitrate.
From www.numerade.com
SOLVED 'When silver nitrate reacts with barium chloride; silver Barium Bromide Reacts With Silver Nitrate Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. Find out what type of reaction occured. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling.. Barium Bromide Reacts With Silver Nitrate.
From www.youtube.com
Reaction between Silver Nitrate and Barium Chloride YouTube Barium Bromide Reacts With Silver Nitrate The net ionic equation for this. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and. Barium Bromide Reacts With Silver Nitrate.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Barium Bromide Reacts With Silver Nitrate Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Find out what type of reaction occured. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide). Barium Bromide Reacts With Silver Nitrate.
From www.numerade.com
SOLVED Complete the table below by deciding whether a precipitate Barium Bromide Reacts With Silver Nitrate Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). The net ionic equation for this. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! Two important uses of precipitation reactions are to. Barium Bromide Reacts With Silver Nitrate.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Barium Bromide Reacts With Silver Nitrate This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. Solutions of sodium chloride and. Barium Bromide Reacts With Silver Nitrate.
From www.youtube.com
Sodium bromide YouTube Barium Bromide Reacts With Silver Nitrate Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. The net ionic equation for this. Balance any equation or reaction using this chemical equation balancer! Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). This page describes and explains the. Barium Bromide Reacts With Silver Nitrate.
From slideplayer.com
Predicting Products and The Activity Series ppt download Barium Bromide Reacts With Silver Nitrate Find out what type of reaction occured. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. The net ionic equation for this. Balance any equation or. Barium Bromide Reacts With Silver Nitrate.
From www.coursehero.com
[Solved] Silver nitrate reacts with barium chloride in the following Barium Bromide Reacts With Silver Nitrate Balance any equation or reaction using this chemical equation balancer! Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Find out what type of reaction occured. Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate.. Barium Bromide Reacts With Silver Nitrate.
From www.chegg.com
Solved The following molecular equation represents the Barium Bromide Reacts With Silver Nitrate This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Balance any equation or reaction using this chemical equation balancer! Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). The net ionic equation for this. Solutions of sodium chloride and silver nitrate. Barium Bromide Reacts With Silver Nitrate.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector Barium Bromide Reacts With Silver Nitrate Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). The net ionic equation for this. Two important uses of precipitation reactions are to. Barium Bromide Reacts With Silver Nitrate.
From www.chemkits.eu
Potassium bromide, 98.5, 7758023 Barium Bromide Reacts With Silver Nitrate Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! Consider the reaction when aqueous solutions. Barium Bromide Reacts With Silver Nitrate.
From www.flinnsci.ca
Sodium Bromide, Reagent, 100 g Flinn Scientific Barium Bromide Reacts With Silver Nitrate Balance any equation or reaction using this chemical equation balancer! Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by. Barium Bromide Reacts With Silver Nitrate.
From www.drsebiscellfood.com
Bromide Plus Powder Dr. Sebi's Cell Food Barium Bromide Reacts With Silver Nitrate Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). Balance any equation or reaction using this chemical equation balancer! The net ionic equation for this. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Solutions of sodium chloride and silver nitrate. Barium Bromide Reacts With Silver Nitrate.
From www.numerade.com
SOLVED DATA TABLE Reaction Observations silver nitrate sodium chloride Barium Bromide Reacts With Silver Nitrate Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. The net ionic equation for this. Find out what type of reaction. Barium Bromide Reacts With Silver Nitrate.
From www.rpicorp.com
E718005.0 Ethidium Bromide, Powder, 5 Grams Barium Bromide Reacts With Silver Nitrate Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! The net ionic equation for this. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Two important uses of precipitation reactions are to isolate metals that have been extracted. Barium Bromide Reacts With Silver Nitrate.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Barium Bromide Reacts With Silver Nitrate Find out what type of reaction occured. The net ionic equation for this. Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. Two important uses of precipitation reactions are to isolate metals that have been. Barium Bromide Reacts With Silver Nitrate.
From www.toppr.com
Write the balanced chemical equation the following reactions & identify Barium Bromide Reacts With Silver Nitrate Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Consider the reaction when aqueous solutions of silver (i) nitrate and barium bromide are combined. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). This page describes and explains the tests. Barium Bromide Reacts With Silver Nitrate.
From sielc.com
Vinyl bromide SIELC Technologies Barium Bromide Reacts With Silver Nitrate Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver. Barium Bromide Reacts With Silver Nitrate.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Barium Bromide Reacts With Silver Nitrate Balance any equation or reaction using this chemical equation balancer! Solutions of sodium chloride and silver nitrate react to form a precipitate of silver chloride and aqueous sodium nitrate. The net ionic equation for this. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Consider. Barium Bromide Reacts With Silver Nitrate.
From nsilabsolutions.com
Bromide CRM IS019 NSI Lab Solutions Barium Bromide Reacts With Silver Nitrate Find out what type of reaction occured. Mixing potassium chromate and silver nitrate together to initiate a precipitation reaction (equation 4.2.1). Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Balance any equation or reaction using this chemical equation balancer! This page describes and explains. Barium Bromide Reacts With Silver Nitrate.