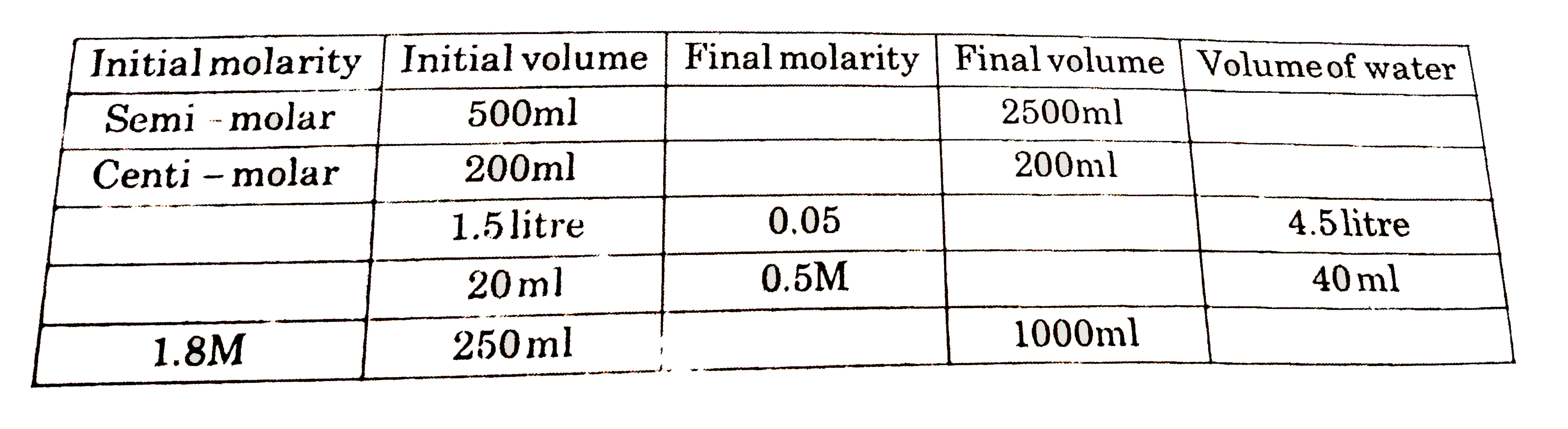
Dilution Basic Question . There’s a bottle of 0.750 m nacl on a shelf. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We are often concerned with how much solute is dissolved in a given amount of. Dilution problems, chemistry, molarity & concentration examples, formula & equations Understand how stock solutions are used in the laboratory. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Dilution m 1v 1=m 2v 2 1. Concentration is the removal of solvent, which increases the. How much of it do you need to prepare 50 ml of a. The dilution equation is a formula used in chemistry to calculate the concentration of a solution after it has been diluted with a solvent.
from www.doubtnut.com
The dilution equation is a formula used in chemistry to calculate the concentration of a solution after it has been diluted with a solvent. How much of it do you need to prepare 50 ml of a. Dilution m 1v 1=m 2v 2 1. Understand how stock solutions are used in the laboratory. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. We are often concerned with how much solute is dissolved in a given amount of. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution problems, chemistry, molarity & concentration examples, formula & equations Concentration is the removal of solvent, which increases the.
Use of dilution formula (M(1)V(1) = M(2) V(2))
Dilution Basic Question There’s a bottle of 0.750 m nacl on a shelf. The dilution equation is a formula used in chemistry to calculate the concentration of a solution after it has been diluted with a solvent. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Concentration is the removal of solvent, which increases the. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution m 1v 1=m 2v 2 1. Understand how stock solutions are used in the laboratory. We are often concerned with how much solute is dissolved in a given amount of. How much of it do you need to prepare 50 ml of a. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Dilution problems, chemistry, molarity & concentration examples, formula & equations Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There’s a bottle of 0.750 m nacl on a shelf.
From www.youtube.com
Dilution Practice Problems & Example Problems YouTube Dilution Basic Question There’s a bottle of 0.750 m nacl on a shelf. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. How much of it do you need to prepare 50 ml of a. Dilution problems, chemistry, molarity & concentration examples, formula & equations Concentration is the removal of solvent, which increases the. A dilution. Dilution Basic Question.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Basic Question Concentration is the removal of solvent, which increases the. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. The dilution equation is a formula used in chemistry to calculate the concentration of a solution after it has been diluted with a solvent. How much of it do you. Dilution Basic Question.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Basic Question Concentration is the removal of solvent, which increases the. Understand how stock solutions are used in the laboratory. Dilution problems, chemistry, molarity & concentration examples, formula & equations Dilution m 1v 1=m 2v 2 1. How much of it do you need to prepare 50 ml of a. There’s a bottle of 0.750 m nacl on a shelf. We are. Dilution Basic Question.
From byjus.com
Dilution law Dilution Basic Question A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution problems, chemistry, molarity & concentration examples, formula & equations There’s a bottle of 0.750 m nacl on a shelf. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the. Dilution Basic Question.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Basic Question Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. How much of it do you need to prepare 50 ml of a. Dilution m 1v. Dilution Basic Question.
From www.youtube.com
Dilutions Explained with Problems YouTube Dilution Basic Question Dilution m 1v 1=m 2v 2 1. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. We are often concerned with how much solute is dissolved in a given amount of. Dilution problems, chemistry, molarity & concentration examples, formula & equations There’s a bottle of 0.750 m. Dilution Basic Question.
From www.chegg.com
Solved Practice Dilution Problems Section I 1. How much Dilution Basic Question Understand how stock solutions are used in the laboratory. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. How much of it do you need to prepare 50 ml of a. A dilution is a process where the concentration of a solution is lowered by adding solvent. Dilution Basic Question.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Basic Question Dilution problems, chemistry, molarity & concentration examples, formula & equations We are often concerned with how much solute is dissolved in a given amount of. Understand how stock solutions are used in the laboratory. Concentration is the removal of solvent, which increases the. Dilution m 1v 1=m 2v 2 1. If you dilute 175 ml of a 1.6 m solution. Dilution Basic Question.
From www.youtube.com
Dilution Calculations How to Use Alligation and Algebraic Methods to Dilution Basic Question Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. There’s a bottle of 0.750 m nacl on a shelf. Dilution m 1v 1=m 2v 2. Dilution Basic Question.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Basic Question Dilution m 1v 1=m 2v 2 1. We are often concerned with how much solute is dissolved in a given amount of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. How much of it do you need to prepare 50 ml of a. A dilution is a process where the concentration of. Dilution Basic Question.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Basic Question Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Understand how stock solutions are used in the laboratory. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. There’s a bottle of 0.750 m nacl on. Dilution Basic Question.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Basic Question Concentration is the removal of solvent, which increases the. We are often concerned with how much solute is dissolved in a given amount of. Understand how stock solutions are used in the laboratory. How much of it do you need to prepare 50 ml of a. There’s a bottle of 0.750 m nacl on a shelf. If you dilute 175. Dilution Basic Question.
From www.doubtnut.com
Use of dilution formula (M(1)V(1) = M(2) V(2)) Dilution Basic Question Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. We are often concerned with how much solute is dissolved in a given amount of. Concentration is the removal of solvent, which increases the. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l,. Dilution Basic Question.
From www.numerade.com
SOLVED 1. Serial DilutionCalculate the Dilution value Final Dilution Dilution Basic Question We are often concerned with how much solute is dissolved in a given amount of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. The dilution equation is a formula used. Dilution Basic Question.
From slidetodoc.com
Dilution of Solutions 1 Volume to volume dilutions Dilution Basic Question A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine. Dilution Basic Question.
From www.studypool.com
SOLUTION Dilution Problems Molarity Formula and Dilution Factor PAper Dilution Basic Question Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Concentration is the removal of solvent, which increases the. How much of it do you need to prepare 50 ml of. Dilution Basic Question.
From www.numerade.com
SOLVED Serial Dilution Worksheet Bio152 Paramedical Microbiology Use Dilution Basic Question Dilution m 1v 1=m 2v 2 1. Dilution problems, chemistry, molarity & concentration examples, formula & equations How much of it do you need to prepare 50 ml of a. Understand how stock solutions are used in the laboratory. Concentration is the removal of solvent, which increases the. A dilution is a process where the concentration of a solution is. Dilution Basic Question.
From www.scribd.com
How to Make Simple Solutions and Dilutions Simple Dilution A Question Dilution Basic Question We are often concerned with how much solute is dissolved in a given amount of. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. How much of it do you need to prepare 50 ml of a. Dilution m 1v 1=m 2v 2 1. Dilution is. Dilution Basic Question.
From www.youtube.com
Dilution Calculations Use This Trick To Easily Solve Dilution of Acids Dilution Basic Question How much of it do you need to prepare 50 ml of a. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. There’s a bottle of 0.750 m nacl on a shelf. Dilution is the addition of solvent, which decreases the concentration of the solute in the. Dilution Basic Question.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilution Basic Question How much of it do you need to prepare 50 ml of a. We are often concerned with how much solute is dissolved in a given amount of. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Dilution is the addition of solvent, which decreases the concentration of. Dilution Basic Question.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Basic Question A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution problems, chemistry, molarity & concentration examples, formula & equations Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. If you dilute 175 ml of a 1.6 m solution of. Dilution Basic Question.
From www.chegg.com
Solved Dilution diagrams can be very helpful in organizing Dilution Basic Question If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. We are often concerned with how much solute is dissolved in a given amount of. There’s a bottle of 0.750 m nacl on a shelf. Understand how stock solutions are used in the laboratory. Dilution m 1v 1=m. Dilution Basic Question.
From www.numerade.com
SOLVED Using the following diagram, answer the questions below 1 Dilution Basic Question We are often concerned with how much solute is dissolved in a given amount of. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. There’s a bottle of 0.750 m nacl on a shelf. If you dilute 175 ml of a 1.6 m solution of licl to 1.0. Dilution Basic Question.
From www.chegg.com
Solved Question 9 1pts The usedilution values for two Dilution Basic Question Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We are often concerned with how much solute is dissolved in a given amount of. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Dilution problems, chemistry, molarity & concentration examples, formula. Dilution Basic Question.
From www.pinterest.com
DILUTIONS M1V1; M2V2 Solving Dilution Problems in Solution Chemistry Dilution Basic Question There’s a bottle of 0.750 m nacl on a shelf. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. A dilution is a process where the concentration of a solution is. Dilution Basic Question.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Basic Question Dilution m 1v 1=m 2v 2 1. Dilution problems, chemistry, molarity & concentration examples, formula & equations A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. There’s a bottle of 0.750 m nacl on a shelf. How much of it do you need to prepare 50. Dilution Basic Question.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Basic Question Understand how stock solutions are used in the laboratory. Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m nacl on a shelf. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Concentration is the removal of solvent, which increases the. Dilution problems,. Dilution Basic Question.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Basic Question Concentration is the removal of solvent, which increases the. Dilution problems, chemistry, molarity & concentration examples, formula & equations How much of it do you need to prepare 50 ml of a. Dilution m 1v 1=m 2v 2 1. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There’s a bottle of 0.750. Dilution Basic Question.
From www.chegg.com
Solved Dilution Problems For problems 711 use the dilution Dilution Basic Question How much of it do you need to prepare 50 ml of a. Dilution m 1v 1=m 2v 2 1. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. A dilution is. Dilution Basic Question.
From www.pinterest.com
Solutions and Molarity Quiz (Solutes, Solvents, Saturation, and Dilution Basic Question If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. How much of it do you need to prepare 50 ml of a. There’s a bottle of 0.750 m nacl on a shelf. Dilution m 1v 1=m 2v 2 1. Concentration is the removal of solvent, which increases. Dilution Basic Question.
From www.chegg.com
Solved Question 16 (1 point) Use the Dilution formula to Dilution Basic Question We are often concerned with how much solute is dissolved in a given amount of. There’s a bottle of 0.750 m nacl on a shelf. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Dilution problems, chemistry, molarity & concentration examples, formula & equations A dilution is a. Dilution Basic Question.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Basic Question Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Concentration is the removal of solvent, which increases the. If you dilute 175 ml of a 1.6 m solution of licl to 1.0. Dilution Basic Question.
From www.chegg.com
Solved Use the dilution scheme shown below and answer the Dilution Basic Question Concentration is the removal of solvent, which increases the. Understand how stock solutions are used in the laboratory. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution problems, chemistry, molarity & concentration examples, formula & equations Dilution is the addition of solvent, which decreases the. Dilution Basic Question.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution Basic Question Understand how stock solutions are used in the laboratory. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. There’s a bottle of 0.750 m nacl on a shelf. Dilution problems, chemistry, molarity & concentration examples, formula & equations The dilution equation is a formula used in chemistry. Dilution Basic Question.
From www.studocu.com
Serial dilution In simple words, serial dilution is the process of Dilution Basic Question Dilution problems, chemistry, molarity & concentration examples, formula & equations Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m. Dilution Basic Question.