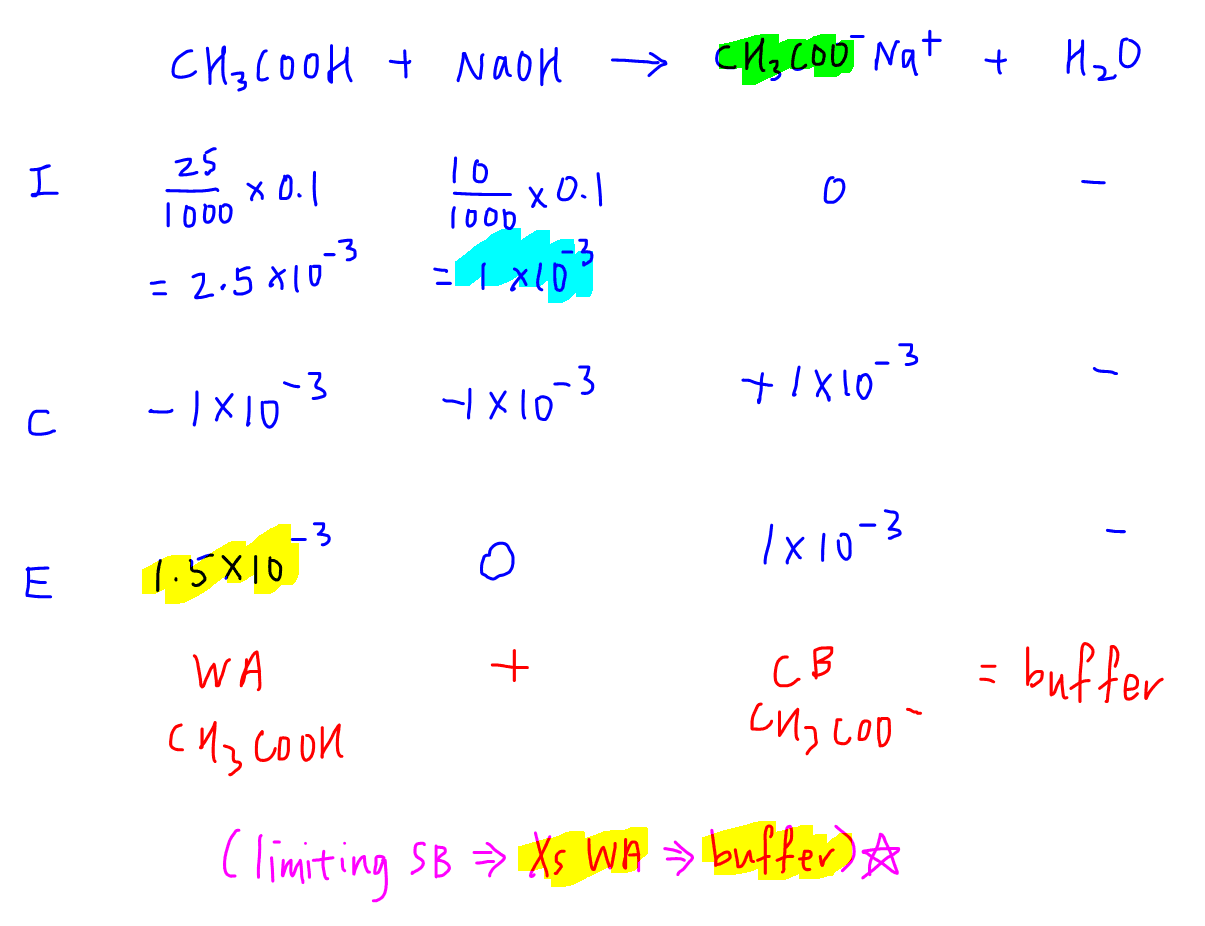Ice Table Buffer . Citric acid is a useful component of a buffer mixture because it has three pk a values, separated by less than two. An ice (i nitial, c hange, e quilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. 50.0 ml of 0.100 m hcl was added to. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. An ice chart is useful in determining the ph of the system after a strong acid has been added. A buffer is created in a solution which contains both a weak acid and its conjugate base. This creates to absorb excess h + or. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. Examples of ph calculations for buffer solutions. The method requires knowing the concentrations of. Let's say you are asked to calculate the ph of a solution with: We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method.
from chemguru.sg
Examples of ph calculations for buffer solutions. A buffer is created in a solution which contains both a weak acid and its conjugate base. 50.0 ml of 0.100 m hcl was added to. We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. An ice chart is useful in determining the ph of the system after a strong acid has been added. An ice (i nitial, c hange, e quilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. The method requires knowing the concentrations of. Let's say you are asked to calculate the ph of a solution with: This creates to absorb excess h + or.
Determine pH of Resultant Solution of AcidBase Reaction
Ice Table Buffer Citric acid is a useful component of a buffer mixture because it has three pk a values, separated by less than two. Let's say you are asked to calculate the ph of a solution with: We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. An ice chart is useful in determining the ph of the system after a strong acid has been added. 50.0 ml of 0.100 m hcl was added to. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. Citric acid is a useful component of a buffer mixture because it has three pk a values, separated by less than two. The method requires knowing the concentrations of. This creates to absorb excess h + or. A buffer is created in a solution which contains both a weak acid and its conjugate base. Examples of ph calculations for buffer solutions. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. An ice (i nitial, c hange, e quilibrium) table is simple matrix formalism that used to simplify the calculations in reversible.
From sfb-foerdertechnik.de
Rotary Tables & Buffer Tables SFB Fördertechnik GmbH Ice Table Buffer 50.0 ml of 0.100 m hcl was added to. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. A buffer is created in a solution which contains both a weak acid and its conjugate base. Examples of ph calculations. Ice Table Buffer.
From general.chemistrysteps.com
The Common Ion Effect Chemistry Steps Ice Table Buffer A buffer is created in a solution which contains both a weak acid and its conjugate base. 50.0 ml of 0.100 m hcl was added to. An ice chart is useful in determining the ph of the system after a strong acid has been added. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is.. Ice Table Buffer.
From studylibmayer.z13.web.core.windows.net
Ice Table Chemistry Worksheet Ice Table Buffer Citric acid is a useful component of a buffer mixture because it has three pk a values, separated by less than two. An ice (i nitial, c hange, e quilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. Let's say you are asked to calculate the ph of a solution with: An ice chart is. Ice Table Buffer.
From elchoroukhost.net
Ice Table Calculator Online Elcho Table Ice Table Buffer We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. An ice (i nitial, c hange, e quilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. Citric acid is a. Ice Table Buffer.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc Membership Ice Table Buffer Let's say you are asked to calculate the ph of a solution with: Examples of ph calculations for buffer solutions. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. A buffer is created in a solution which contains both. Ice Table Buffer.
From www.chegg.com
Solved Complete the ICE table below for the addition of 1.00 Ice Table Buffer This creates to absorb excess h + or. Let's say you are asked to calculate the ph of a solution with: We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. Examples of ph calculations for buffer solutions. How to calculate ph of buffer solutionscalculate the ph of a buffer. Ice Table Buffer.
From www.semanticscholar.org
Measurement of pHT values of Tris buffers in artificial seawater at Ice Table Buffer Examples of ph calculations for buffer solutions. 50.0 ml of 0.100 m hcl was added to. We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. The method requires knowing the concentrations of. Let's say. Ice Table Buffer.
From www.youtube.com
Week 9 9. pH of a buffer using an ICE chart YouTube Ice Table Buffer 50.0 ml of 0.100 m hcl was added to. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. An ice chart is useful in determining the ph of the system after a strong acid has been added. Examples of ph calculations for buffer solutions. The method requires knowing the concentrations of. Citric acid is. Ice Table Buffer.
From www.youtube.com
ICE Tables for a Weak Base and Buffers in General Chemistry YouTube Ice Table Buffer This creates to absorb excess h + or. Examples of ph calculations for buffer solutions. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. An ice (i nitial, c hange, e quilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. Let's say you are asked to calculate the ph. Ice Table Buffer.
From www.plumpuddingchemistry.com
5 key concepts that make up AP Chemistry Acids and Bases Vaishnavi Ice Table Buffer The method requires knowing the concentrations of. Citric acid is a useful component of a buffer mixture because it has three pk a values, separated by less than two. This creates to absorb excess h + or. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. Let's say you are asked to calculate the. Ice Table Buffer.
From www.slideshare.net
CM4106 Review of Lesson 3 (Part 1) Ice Table Buffer 50.0 ml of 0.100 m hcl was added to. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. Let's say you are asked to calculate the ph of a solution with: The method requires knowing the concentrations of. This creates to absorb excess h + or. A buffer is created in a solution which. Ice Table Buffer.
From bluelightballroom.com
Best Ice Tables For Your Next Party Ice Table Buffer How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. An ice chart is useful in determining the ph of the system after a strong acid has been added. A buffer is created in a solution which contains both a weak acid and its conjugate base. This creates to absorb excess h + or. Buffers,. Ice Table Buffer.
From www.walmart.com
Modern Home 5' Portable Folding Party Ice Bin Table with Skirt White Ice Table Buffer Citric acid is a useful component of a buffer mixture because it has three pk a values, separated by less than two. This creates to absorb excess h + or. 50.0 ml of 0.100 m hcl was added to. An ice chart is useful in determining the ph of the system after a strong acid has been added. An ice. Ice Table Buffer.
From zjxczl.en.made-in-china.com
Stainless Frozen Food Ice Table Freezer with Open Area for Seafood Ice Table Buffer We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. Citric acid is a useful component of a buffer mixture because it has three pk a values, separated by less than two. A buffer is. Ice Table Buffer.
From www.chegg.com
Solved Determine the concentration of C6H5NH3+in a buffer Ice Table Buffer Citric acid is a useful component of a buffer mixture because it has three pk a values, separated by less than two. 50.0 ml of 0.100 m hcl was added to. A buffer is created in a solution which contains both a weak acid and its conjugate base. How to calculate ph of buffer solutionscalculate the ph of a buffer. Ice Table Buffer.
From www.youtube.com
Finding pH of a buffer system using ICE table YouTube Ice Table Buffer A buffer is created in a solution which contains both a weak acid and its conjugate base. Examples of ph calculations for buffer solutions. 50.0 ml of 0.100 m hcl was added to. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. An ice chart is useful in determining the ph of the system. Ice Table Buffer.
From www.showme.com
ShowMe Ice table Ice Table Buffer How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. Examples of ph calculations for buffer solutions. We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. Let's say you are asked to calculate the ph of a solution with: Citric acid is a useful. Ice Table Buffer.
From chemistry.stackexchange.com
chemistry Understanding how to calculate the pH of a buffer Ice Table Buffer How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. An ice (i nitial, c hange, e quilibrium) table is. Ice Table Buffer.
From quizinterworks.z21.web.core.windows.net
How To Use Ice Tables Ice Table Buffer We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. 50.0 ml of 0.100 m hcl was added to. Citric acid is a useful component of a buffer mixture because it has three pk a values, separated by less than two. How to calculate ph of buffer solutionscalculate the ph. Ice Table Buffer.
From www.amazon.ca
Giantex 4 Foot Ice Cooler Table w/ Matching Skirt and Drain Plug Ice Table Buffer This creates to absorb excess h + or. A buffer is created in a solution which contains both a weak acid and its conjugate base. An ice (i nitial, c hange, e quilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that. Ice Table Buffer.
From www.chegg.com
Solved Determine the pH of a buffer solution by constructing Ice Table Buffer An ice chart is useful in determining the ph of the system after a strong acid has been added. We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. 50.0 ml of 0.100 m hcl was added to. Let's say you are asked to calculate the ph of a solution. Ice Table Buffer.
From vandue.com
Modern Home 4' Party Ice Bin Table with Skirt Portable Folding Ice Table Buffer Examples of ph calculations for buffer solutions. We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. The method requires knowing the concentrations of. An ice chart is useful in determining the ph of the. Ice Table Buffer.
From www.northstarice.com
Buffer Tanks & Case Ice Dispensers North Star Best Commercial Ice Table Buffer An ice chart is useful in determining the ph of the system after a strong acid has been added. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. Examples of ph calculations for buffer solutions. Citric acid is a. Ice Table Buffer.
From www.youtube.com
Biochemistry Buffers ICE Problem With HendersonHasselbalch Ice Table Buffer Let's say you are asked to calculate the ph of a solution with: An ice chart is useful in determining the ph of the system after a strong acid has been added. 50.0 ml of 0.100 m hcl was added to. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. A buffer is created. Ice Table Buffer.
From www.slideserve.com
PPT Equilibrium Acids and Bases PowerPoint Presentation, free Ice Table Buffer 50.0 ml of 0.100 m hcl was added to. The method requires knowing the concentrations of. This creates to absorb excess h + or. An ice (i nitial, c hange, e quilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. A buffer is created in a solution which contains both a weak acid and its. Ice Table Buffer.
From www.youtube.com
VINGLI 4FT Party Ice Cooler Table Foldable Camping Buffet Ice Table Ice Table Buffer Examples of ph calculations for buffer solutions. A buffer is created in a solution which contains both a weak acid and its conjugate base. The method requires knowing the concentrations of. Citric acid is a useful component of a buffer mixture because it has three pk a values, separated by less than two. Buffers, or buffered solutions, resist changes in. Ice Table Buffer.
From elchoroukhost.net
Ice Table Calculator Online Elcho Table Ice Table Buffer The method requires knowing the concentrations of. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. 50.0 ml of 0.100 m hcl was added to. An ice (i nitial, c hange, e quilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. Citric acid is a useful component of a. Ice Table Buffer.
From www.chegg.com
Solved Determine the pH of a buffer solution by constructing Ice Table Buffer The method requires knowing the concentrations of. How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. An ice (i nitial, c hange, e quilibrium) table is simple matrix formalism that used to simplify the calculations in reversible. 50.0 ml of 0.100 m hcl was added to. An ice chart is useful in determining the. Ice Table Buffer.
From www.youtube.com
InitialChangeEquilibrium (ICE) Tables YouTube Ice Table Buffer Let's say you are asked to calculate the ph of a solution with: We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. A buffer is created in a solution which contains both a weak acid and its conjugate base. 50.0 ml of 0.100 m hcl was added to. How. Ice Table Buffer.
From www.pinterest.com
Giantex Folding Ice Tables for Parties with Drain, Fill and Chill Table Ice Table Buffer The method requires knowing the concentrations of. Citric acid is a useful component of a buffer mixture because it has three pk a values, separated by less than two. 50.0 ml of 0.100 m hcl was added to. Examples of ph calculations for buffer solutions. Let's say you are asked to calculate the ph of a solution with: Buffers, or. Ice Table Buffer.
From ar.inspiredpencil.com
Five Percent Rule Chemistry Ice Table Buffer How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. Buffers, or buffered solutions, resist changes in ph upon the addition of acid or base. A buffer is created in a solution which contains both a weak acid and its conjugate base. An ice chart is useful in determining the ph of the system after. Ice Table Buffer.
From www.zimtown.com
Zimtown 4 Foot Folding Ice Cooler Table, with Matching Skirt and Drain Ice Table Buffer Let's say you are asked to calculate the ph of a solution with: 50.0 ml of 0.100 m hcl was added to. Citric acid is a useful component of a buffer mixture because it has three pk a values, separated by less than two. An ice (i nitial, c hange, e quilibrium) table is simple matrix formalism that used to. Ice Table Buffer.
From chemguru.sg
Determine pH of Resultant Solution of AcidBase Reaction Ice Table Buffer We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. An ice chart is useful in determining the ph of the system after a strong acid has been added. 50.0 ml of 0.100 m hcl was added to. A buffer is created in a solution which contains both a weak. Ice Table Buffer.
From www.chegg.com
Solved 6. From the buffer table below Circle the buffer Ice Table Buffer A buffer is created in a solution which contains both a weak acid and its conjugate base. This creates to absorb excess h + or. The method requires knowing the concentrations of. We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. An ice chart is useful in determining the. Ice Table Buffer.
From www.chegg.com
(Buffers) Fill the following tables using the ICE Ice Table Buffer We have seen in example \(\pageindex{1}\) how the ph of a buffer may be calculated using the ice table method. 50.0 ml of 0.100 m hcl was added to. Let's say you are asked to calculate the ph of a solution with: How to calculate ph of buffer solutionscalculate the ph of a buffer solution that is. Citric acid is. Ice Table Buffer.