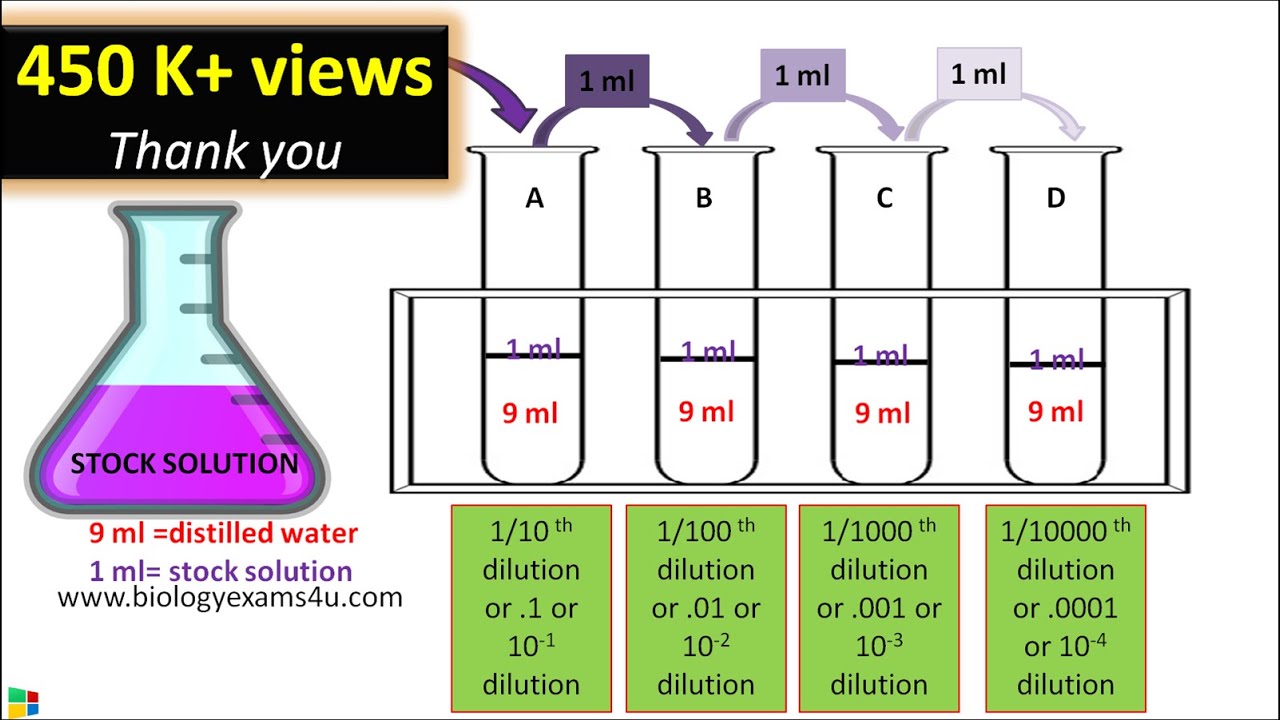
How To Do Dilutions Chemistry . We can relate the concentrations and volumes before and after a dilution using the following. follow these five steps to make a dilution: learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. start practicing—and saving your progress—now! you can calculate the concentration of a solution following a dilution by applying this equation: If you do not know them already, use the dilution factor. Of course, the resulting solution is. to dilute a solution means to add more solvent without the addition of more solute. this process is known as dilution. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure. learn how to dilute solutions. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values.
from www.youtube.com
If you do not know them already, use the dilution factor. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how to dilute solutions. learn how to dilute and concentrate solutions. this process is known as dilution. We can relate the concentrations and volumes before and after a dilution using the following. you can calculate the concentration of a solution following a dilution by applying this equation: start practicing—and saving your progress—now! follow these five steps to make a dilution:
Serial Dilution Method Protocol Step Wise Explanation YouTube
How To Do Dilutions Chemistry you can calculate the concentration of a solution following a dilution by applying this equation: start practicing—and saving your progress—now! learn how to dilute and concentrate solutions. Of course, the resulting solution is. you can calculate the concentration of a solution following a dilution by applying this equation: We can relate the concentrations and volumes before and after a dilution using the following. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. learn how to dilute solutions. to dilute a solution means to add more solvent without the addition of more solute. If you do not know them already, use the dilution factor. this process is known as dilution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure. follow these five steps to make a dilution:
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions How To Do Dilutions Chemistry Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure. Of course, the resulting solution is. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. you can calculate the concentration of a solution. How To Do Dilutions Chemistry.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate How To Do Dilutions Chemistry We can relate the concentrations and volumes before and after a dilution using the following. you can calculate the concentration of a solution following a dilution by applying this equation: Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Solutions can be prepared by diluting a more concentrated solution rather. How To Do Dilutions Chemistry.
From www.youtube.com
Beginning Chemistry Solution Dilution YouTube How To Do Dilutions Chemistry Often, a worker will need to change the concentration of a solution by changing the amount of solvent. We can relate the concentrations and volumes before and after a dilution using the following. Of course, the resulting solution is. to dilute a solution means to add more solvent without the addition of more solute. learn how to dilute. How To Do Dilutions Chemistry.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube How To Do Dilutions Chemistry We can relate the concentrations and volumes before and after a dilution using the following. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how to dilute solutions. follow these five steps to make a dilution: If you do not know them already, use the dilution factor. M. How To Do Dilutions Chemistry.
From dxohqxlgl.blob.core.windows.net
Dilution 1 Volume at Jeremiah Gonzalez blog How To Do Dilutions Chemistry to dilute a solution means to add more solvent without the addition of more solute. follow these five steps to make a dilution: M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. If you do not know them. How To Do Dilutions Chemistry.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples How To Do Dilutions Chemistry Often, a worker will need to change the concentration of a solution by changing the amount of solvent. this process is known as dilution. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure. M i v i = m f v f where m is molarity, v is volume, and the subscripts. How To Do Dilutions Chemistry.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts How To Do Dilutions Chemistry learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. follow these five steps to make a dilution: We can relate the concentrations and volumes before and after a dilution using the following. Solutions can be prepared by diluting a more concentrated solution. How To Do Dilutions Chemistry.
From www.wikihow.com
How to Do Serial Dilutions 9 Steps (with Pictures) wikiHow How To Do Dilutions Chemistry this process is known as dilution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. We can relate the concentrations and volumes before and after a dilution using the following. learn how to dilute and concentrate solutions. Of course, the resulting solution is. If you do not know them. How To Do Dilutions Chemistry.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query How To Do Dilutions Chemistry We can relate the concentrations and volumes before and after a dilution using the following. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and. How To Do Dilutions Chemistry.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 How To Do Dilutions Chemistry If you do not know them already, use the dilution factor. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. start practicing—and saving your progress—now! to dilute a solution means to add more solvent without the addition of. How To Do Dilutions Chemistry.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The How To Do Dilutions Chemistry this process is known as dilution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. start practicing—and saving your progress—now! If you do not know them already, use the dilution factor. M i v i = m f v f where m is molarity, v is volume, and the. How To Do Dilutions Chemistry.
From www.pinterest.cl
Dilution when solvent is added to dilute a solution, the number of How To Do Dilutions Chemistry Of course, the resulting solution is. start practicing—and saving your progress—now! Often, a worker will need to change the concentration of a solution by changing the amount of solvent. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. . How To Do Dilutions Chemistry.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube How To Do Dilutions Chemistry to dilute a solution means to add more solvent without the addition of more solute. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure. start practicing—and saving your progress—now! this process is known as dilution. follow these five steps to make a dilution: you can calculate the concentration. How To Do Dilutions Chemistry.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube How To Do Dilutions Chemistry to dilute a solution means to add more solvent without the addition of more solute. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure. start practicing—and saving your progress—now! learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing. How To Do Dilutions Chemistry.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube How To Do Dilutions Chemistry If you do not know them already, use the dilution factor. learn how to dilute solutions. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. Of course, the resulting solution is. Solutions can be prepared by diluting a more. How To Do Dilutions Chemistry.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog How To Do Dilutions Chemistry to dilute a solution means to add more solvent without the addition of more solute. you can calculate the concentration of a solution following a dilution by applying this equation: learn how to dilute and concentrate solutions. learn how to dilute solutions. We can relate the concentrations and volumes before and after a dilution using the. How To Do Dilutions Chemistry.
From www.showme.com
Dilution calculation Science, Chemicalreactions, Biology ShowMe How To Do Dilutions Chemistry you can calculate the concentration of a solution following a dilution by applying this equation: M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. Often, a worker will need to change the concentration of a solution by changing the. How To Do Dilutions Chemistry.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube How To Do Dilutions Chemistry you can calculate the concentration of a solution following a dilution by applying this equation: start practicing—and saving your progress—now! to dilute a solution means to add more solvent without the addition of more solute. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how to. How To Do Dilutions Chemistry.
From www.slideserve.com
PPT Making Dilutions & Electrolytes PowerPoint Presentation ID6829039 How To Do Dilutions Chemistry Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. to dilute a solution means to add more solvent without the addition of more. How To Do Dilutions Chemistry.
From www.actioncleanup.com
Dilution Action’s Guide to Mixing the Right Solutions How To Do Dilutions Chemistry We can relate the concentrations and volumes before and after a dilution using the following. you can calculate the concentration of a solution following a dilution by applying this equation: start practicing—and saving your progress—now! this process is known as dilution. Often, a worker will need to change the concentration of a solution by changing the amount. How To Do Dilutions Chemistry.
From www.youtube.com
Dilution Calculation Practice YouTube How To Do Dilutions Chemistry If you do not know them already, use the dilution factor. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. to dilute a solution means to add more solvent without the addition of more solute. Often, a worker will. How To Do Dilutions Chemistry.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and How To Do Dilutions Chemistry start practicing—and saving your progress—now! learn how to dilute solutions. to dilute a solution means to add more solvent without the addition of more solute. follow these five steps to make a dilution: you can calculate the concentration of a solution following a dilution by applying this equation: learn how to dilute and concentrate. How To Do Dilutions Chemistry.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in How To Do Dilutions Chemistry this process is known as dilution. Of course, the resulting solution is. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. learn how to dilute solutions. follow these five steps to make a dilution: M i v i = m f v f where m is molarity, v. How To Do Dilutions Chemistry.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality How To Do Dilutions Chemistry this process is known as dilution. you can calculate the concentration of a solution following a dilution by applying this equation: to dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is. M i v i = m f v f where m is molarity, v is. How To Do Dilutions Chemistry.
From materialschoolpinder.z21.web.core.windows.net
How To Do Dilutions In Chemistry How To Do Dilutions Chemistry If you do not know them already, use the dilution factor. follow these five steps to make a dilution: M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. Of course, the resulting solution is. Often, a worker will need. How To Do Dilutions Chemistry.
From dxorvxlft.blob.core.windows.net
Serial Dilutions Lab at Joseph Brown blog How To Do Dilutions Chemistry learn how to dilute and concentrate solutions. you can calculate the concentration of a solution following a dilution by applying this equation: M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. start practicing—and saving your progress—now! . How To Do Dilutions Chemistry.
From mmerevise.co.uk
Concentrations and Dilutions MME How To Do Dilutions Chemistry If you do not know them already, use the dilution factor. learn how to dilute and concentrate solutions. start practicing—and saving your progress—now! this process is known as dilution. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final. How To Do Dilutions Chemistry.
From spmchemistry.blog.onlinetuition.com.my
Dilution SPM Chemistry How To Do Dilutions Chemistry start practicing—and saving your progress—now! Of course, the resulting solution is. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. We can relate the concentrations and volumes before and after a dilution using the following. you can calculate. How To Do Dilutions Chemistry.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples How To Do Dilutions Chemistry start practicing—and saving your progress—now! Of course, the resulting solution is. We can relate the concentrations and volumes before and after a dilution using the following. you can calculate the concentration of a solution following a dilution by applying this equation: learn how to dilute solutions. Often, a worker will need to change the concentration of a. How To Do Dilutions Chemistry.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts How To Do Dilutions Chemistry If you do not know them already, use the dilution factor. to dilute a solution means to add more solvent without the addition of more solute. learn how to dilute and concentrate solutions. this process is known as dilution. follow these five steps to make a dilution: you can calculate the concentration of a solution. How To Do Dilutions Chemistry.
From exoaldujs.blob.core.windows.net
How To Write Dilutions at Judy Moody blog How To Do Dilutions Chemistry If you do not know them already, use the dilution factor. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure. learn how to dilute and concentrate solutions. learn how to dilute solutions. Of course, the resulting solution is. you can calculate the concentration of a solution following a dilution by. How To Do Dilutions Chemistry.
From www.youtube.com
Serial Dilution Methods & Calaculations YouTube How To Do Dilutions Chemistry start practicing—and saving your progress—now! to dilute a solution means to add more solvent without the addition of more solute. If you do not know them already, use the dilution factor. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure. We can relate the concentrations and volumes before and after a. How To Do Dilutions Chemistry.
From www.youtube.com
Dilution Chemistry How to Calculate and Perform Molarity Dilutions How To Do Dilutions Chemistry M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure. learn how to dilute solutions. you can calculate the concentration of a solution. How To Do Dilutions Chemistry.
From materialschoolpinder.z21.web.core.windows.net
How To Do Dilutions In Chemistry How To Do Dilutions Chemistry Of course, the resulting solution is. learn how to dilute solutions. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure. follow these five steps to make a dilution: We can relate the concentrations and volumes before and after a dilution using the following. learn how to dilute and concentrate solutions.. How To Do Dilutions Chemistry.
From www.wikihow.com
How to Do Serial Dilutions 9 Steps (with Pictures) wikiHow How To Do Dilutions Chemistry M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. follow these five steps to make a dilution: learn how to dilute solutions. Often, a worker will need to change the concentration of a solution by changing the amount. How To Do Dilutions Chemistry.