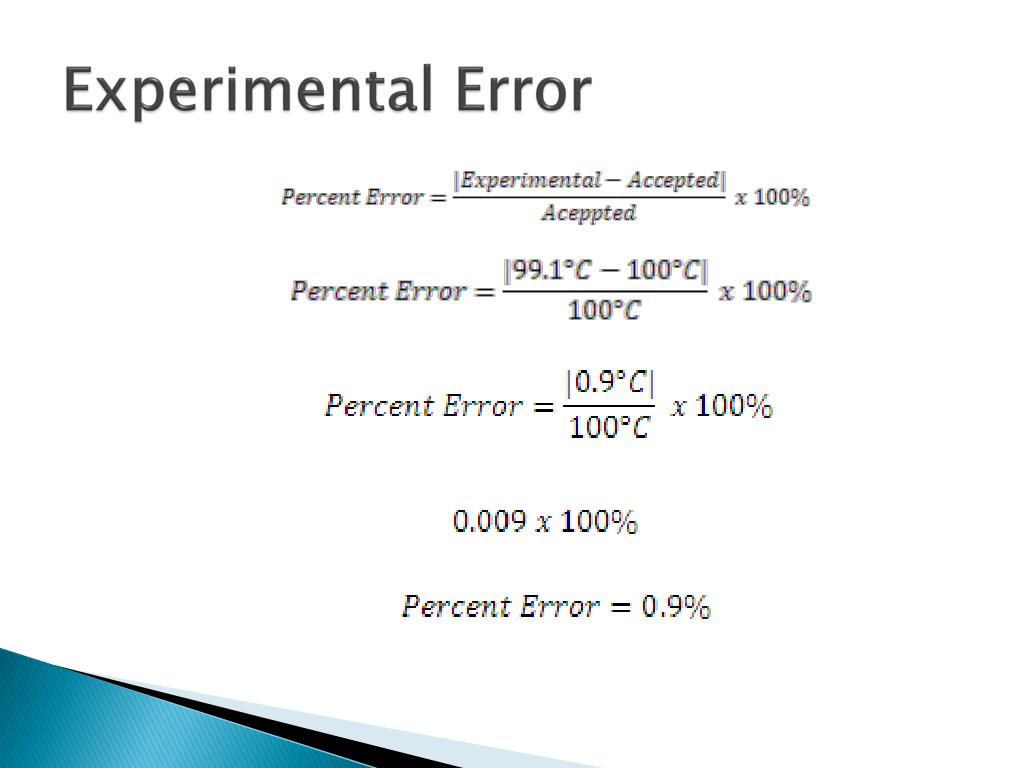
Error Chemistry Easy Definition . If the error in the analysis is large,. What is the difference between random and systematic error? Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). “error” in chemistry is defined as the difference between the true result (or accepted true result) and the measured result. There are two concepts we need to understand in. The amount of error that is acceptable. Random errors are unavoidable but cluster around the true value. Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. Random error introduces variability between different measurements of the same thing, while systematic error skews your measurement away from the true value in a. The difference between your results and the expected or theoretical results is called error.
from www.slideserve.com
Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. “error” in chemistry is defined as the difference between the true result (or accepted true result) and the measured result. If the error in the analysis is large,. Random error introduces variability between different measurements of the same thing, while systematic error skews your measurement away from the true value in a. What is the difference between random and systematic error? There are two concepts we need to understand in. Random errors are unavoidable but cluster around the true value. The amount of error that is acceptable. The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\).
PPT Introduction to Chemistry & Experimental Error PowerPoint Presentation ID6251000
Error Chemistry Easy Definition Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). Random error introduces variability between different measurements of the same thing, while systematic error skews your measurement away from the true value in a. There are two concepts we need to understand in. Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. “error” in chemistry is defined as the difference between the true result (or accepted true result) and the measured result. The difference between your results and the expected or theoretical results is called error. If the error in the analysis is large,. Random errors are unavoidable but cluster around the true value. Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. What is the difference between random and systematic error? The amount of error that is acceptable.
From www.youtube.com
Analytical Chemistry 1 Lecture 3 Error propagation in arithmetic calculations (Slid 6266) YouTube Error Chemistry Easy Definition Random errors are unavoidable but cluster around the true value. Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. The amount of error that is acceptable. Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. Random error introduces variability between different measurements. Error Chemistry Easy Definition.
From eduinput.com
Error in MeasurementDefinition, Types, And Reduction Error Chemistry Easy Definition The difference between your results and the expected or theoretical results is called error. “error” in chemistry is defined as the difference between the true result (or accepted true result) and the measured result. Random errors are unavoidable but cluster around the true value. The percent error is the absolute value of the error, divided by the accepted value, and. Error Chemistry Easy Definition.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID5755545 Error Chemistry Easy Definition Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. Random error introduces variability between. Error Chemistry Easy Definition.
From sciencenotes.org
Absolute and Relative Error and How to Calculate Them Error Chemistry Easy Definition Random error introduces variability between different measurements of the same thing, while systematic error skews your measurement away from the true value in a. Random errors are unavoidable but cluster around the true value. The amount of error that is acceptable. If the error in the analysis is large,. “error” in chemistry is defined as the difference between the true. Error Chemistry Easy Definition.
From www.slideserve.com
PPT Introduction to experimental errors PowerPoint Presentation, free download ID3568686 Error Chemistry Easy Definition Random error introduces variability between different measurements of the same thing, while systematic error skews your measurement away from the true value in a. The amount of error that is acceptable. Random errors are unavoidable but cluster around the true value. Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. If the. Error Chemistry Easy Definition.
From www.slideshare.net
Accuracy Random and Systematic Errors Error Chemistry Easy Definition Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. Random errors are unavoidable but cluster around the true value. The amount of error that is acceptable. The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). Random error introduces variability between different measurements. Error Chemistry Easy Definition.
From www.showme.com
Percent Error chemistry Science, Chemistry, Measurements and Calculations ShowMe Error Chemistry Easy Definition The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. The difference between your results. Error Chemistry Easy Definition.
From www.showme.com
Percent error part 2 Chemistry, Science, Stoichiometry ShowMe Error Chemistry Easy Definition If the error in the analysis is large,. The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. Random errors are unavoidable but cluster around the true value. The difference between your results and. Error Chemistry Easy Definition.
From materialschwartz.z19.web.core.windows.net
Percent Error Worksheet Chemistry Error Chemistry Easy Definition The difference between your results and the expected or theoretical results is called error. Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. Random error introduces variability between different measurements of the same thing, while systematic error skews your measurement away from the true value in a. Random errors are unavoidable but. Error Chemistry Easy Definition.
From www.youtube.com
Analytical Chemistry Indeterminate Error Absolute ErrorsMean ErrorsRelative Errors Saad Error Chemistry Easy Definition The difference between your results and the expected or theoretical results is called error. Random errors are unavoidable but cluster around the true value. There are two concepts we need to understand in. If the error in the analysis is large,. Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments.. Error Chemistry Easy Definition.
From ar.inspiredpencil.com
Percent Error Formula Chemistry Error Chemistry Easy Definition “error” in chemistry is defined as the difference between the true result (or accepted true result) and the measured result. What is the difference between random and systematic error? The amount of error that is acceptable. Random errors are unavoidable but cluster around the true value. Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it. Error Chemistry Easy Definition.
From sciencenotes.org
Systematic vs Random Error Differences and Examples Error Chemistry Easy Definition The difference between your results and the expected or theoretical results is called error. Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. The amount of error that is acceptable. There are two concepts we need to understand in. If the error in the analysis is large,. Random error introduces. Error Chemistry Easy Definition.
From www.expii.com
Types of Error — Overview & Comparison Expii Error Chemistry Easy Definition If the error in the analysis is large,. What is the difference between random and systematic error? The amount of error that is acceptable. Random errors are unavoidable but cluster around the true value. “error” in chemistry is defined as the difference between the true result (or accepted true result) and the measured result. Errors are common occurrences in chemistry. Error Chemistry Easy Definition.
From www.youtube.com
Introduction to Error Analysis for Chemistry Lab YouTube Error Chemistry Easy Definition The amount of error that is acceptable. The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). “error” in chemistry is defined as the difference between the true result (or accepted true result) and the measured result. If the error in the analysis is large,. The difference between your results and. Error Chemistry Easy Definition.
From howtohacks92.blogspot.com
Calculate Percent Error Chemistry Equation For Percent Change In Mass Tessshebaylo / The Error Chemistry Easy Definition The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. There are two concepts we need to understand in. “error” in chemistry is defined as the difference between the true result (or. Error Chemistry Easy Definition.
From www.youtube.com
Spotting procedural errors in chemistry practicals YouTube Error Chemistry Easy Definition Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. Random errors are unavoidable but cluster around the true value. What is the difference between random and systematic error? If the error in the analysis is large,. The amount of error that is acceptable. There are two concepts we need to understand in.. Error Chemistry Easy Definition.
From study.com
How to Calculate Relative Error Chemistry Error Chemistry Easy Definition Random errors are unavoidable but cluster around the true value. What is the difference between random and systematic error? If the error in the analysis is large,. There are two concepts we need to understand in. Random error introduces variability between different measurements of the same thing, while systematic error skews your measurement away from the true value in a.. Error Chemistry Easy Definition.
From chemistnotes.com
Errors in Chemical Analysis Determinate and Indeterminate Errors Chemistry Notes Error Chemistry Easy Definition Random error introduces variability between different measurements of the same thing, while systematic error skews your measurement away from the true value in a. Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. “error” in chemistry is defined as the difference between the true result (or accepted true result) and. Error Chemistry Easy Definition.
From math.wonderhowto.com
How to Calculate percent error in chemistry lab activities « Math WonderHowTo Error Chemistry Easy Definition The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). There are two concepts we need to understand in. “error” in chemistry is defined as the difference between the true result (or accepted true result) and the measured result. Errors are common occurrences in chemistry and there are three specific types. Error Chemistry Easy Definition.
From www.pharmaacademias.com
Sources and Types of Errors in Pharmaceutical Chemistry Pharmaacademias Error Chemistry Easy Definition Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). Random errors are unavoidable but cluster around the true value. The amount of error that is acceptable. Systematic error can often be. Error Chemistry Easy Definition.
From www.youtube.com
Error Definition & Types Chapter 1 Pharmaceutical ChemistryI D.Pharm /Analysis B.Pharm 1st Error Chemistry Easy Definition The amount of error that is acceptable. If the error in the analysis is large,. Random errors are unavoidable but cluster around the true value. The difference between your results and the expected or theoretical results is called error. “error” in chemistry is defined as the difference between the true result (or accepted true result) and the measured result. Errors. Error Chemistry Easy Definition.
From www.sliderbase.com
Significant Figures Presentation Chemistry Error Chemistry Easy Definition Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. If the error in the analysis is large,. What is the difference between random and systematic error? Random errors are unavoidable but cluster around the true value. The percent error is the absolute value of the error, divided by the accepted value, and. Error Chemistry Easy Definition.
From www.vrogue.co
How To Calculate Relative Error Chemistry Study Com vrogue.co Error Chemistry Easy Definition The difference between your results and the expected or theoretical results is called error. Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. The percent error is the absolute value of the error,. Error Chemistry Easy Definition.
From chemistnotes.com
Errors in Chemical Analysis Determinate and Indeterminate Errors Chemistry Notes Error Chemistry Easy Definition Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. The amount of error that is acceptable. Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. The percent error is the absolute value of the error, divided by the accepted value, and multiplied. Error Chemistry Easy Definition.
From chemistnotes.com
Errors in Chemical Analysis Determinate and Indeterminate Errors Chemistry Notes Error Chemistry Easy Definition If the error in the analysis is large,. Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. Random error introduces variability between different measurements of the same thing, while systematic error skews your measurement away from the true value in a. The difference between your results and the expected or. Error Chemistry Easy Definition.
From www.youtube.com
Analytical Chemistry 1 CH5 L2 Types of Error in Experimental Data (Slid 4243) YouTube Error Chemistry Easy Definition Random error introduces variability between different measurements of the same thing, while systematic error skews your measurement away from the true value in a. The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). Random errors are unavoidable but cluster around the true value. If the error in the analysis is. Error Chemistry Easy Definition.
From www.techyv.com
Different Experimental Error Chemistry Student Faces During Titration Error Chemistry Easy Definition The amount of error that is acceptable. The difference between your results and the expected or theoretical results is called error. The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). Random error introduces variability between different measurements of the same thing, while systematic error skews your measurement away from the. Error Chemistry Easy Definition.
From www.expii.com
Types of Error — Overview & Comparison Expii Error Chemistry Easy Definition “error” in chemistry is defined as the difference between the true result (or accepted true result) and the measured result. What is the difference between random and systematic error? Random error introduces variability between different measurements of the same thing, while systematic error skews your measurement away from the true value in a. Random errors are unavoidable but cluster around. Error Chemistry Easy Definition.
From www.youtube.com
11.1 Random and systematic errors (SL) YouTube Error Chemistry Easy Definition The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. There are two concepts we need to understand in. The difference between your results and the expected or theoretical results is called error. Random. Error Chemistry Easy Definition.
From www.slideserve.com
PPT Introduction to Chemistry & Experimental Error PowerPoint Presentation ID6251000 Error Chemistry Easy Definition Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. The difference between your results and the expected or theoretical results is called error. Random errors are unavoidable but cluster around the true value. There are two concepts we need to understand in. Random error introduces variability between different measurements of. Error Chemistry Easy Definition.
From www.slideserve.com
PPT Experimental Error PowerPoint Presentation, free download ID9290012 Error Chemistry Easy Definition There are two concepts we need to understand in. If the error in the analysis is large,. The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). The amount of error that is acceptable. Random error introduces variability between different measurements of the same thing, while systematic error skews your measurement. Error Chemistry Easy Definition.
From www.youtube.com
Measurement Error [ TYPES OF ERROR ] Difference between Systematic Error Vrs Random Error YouTube Error Chemistry Easy Definition Errors are common occurrences in chemistry and there are three specific types of errors that may occur during experiments. There are two concepts we need to understand in. “error” in chemistry is defined as the difference between the true result (or accepted true result) and the measured result. If the error in the analysis is large,. Random error introduces variability. Error Chemistry Easy Definition.
From www.slideshare.net
Errors in chemical analysis Error Chemistry Easy Definition What is the difference between random and systematic error? There are two concepts we need to understand in. The percent error is the absolute value of the error, divided by the accepted value, and multiplied by \(100\%\). Random error introduces variability between different measurements of the same thing, while systematic error skews your measurement away from the true value in. Error Chemistry Easy Definition.
From www.slideserve.com
PPT Introduction to Chemistry & Experimental Error PowerPoint Presentation ID6251000 Error Chemistry Easy Definition The amount of error that is acceptable. Random errors are unavoidable but cluster around the true value. What is the difference between random and systematic error? “error” in chemistry is defined as the difference between the true result (or accepted true result) and the measured result. The percent error is the absolute value of the error, divided by the accepted. Error Chemistry Easy Definition.
From ar.inspiredpencil.com
Percent Error Formula Chemistry Error Chemistry Easy Definition The difference between your results and the expected or theoretical results is called error. “error” in chemistry is defined as the difference between the true result (or accepted true result) and the measured result. The amount of error that is acceptable. Systematic error can often be avoided by calibrating equipment, but if left uncorrected, it can lead to. What is. Error Chemistry Easy Definition.