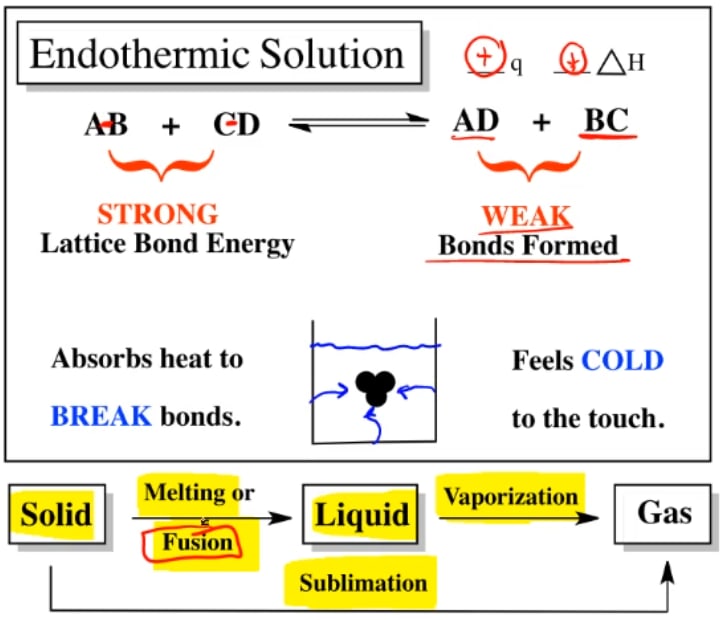
Endothermic Solutions . Because heat is absorbed, endothermic reactions feel cold. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form, although perhaps limiting the. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless.
from www.clutchprep.com
The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form, although perhaps limiting the. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions feel cold. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see.
Endothermic & Exothermic Reactions Chemistry Video Clutch Prep
Endothermic Solutions Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Because heat is absorbed, endothermic reactions feel cold. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form, although perhaps limiting the. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see.
From shaunmwilliams.com
Chapter 11 Presentation Endothermic Solutions The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Because heat is absorbed, endothermic. Endothermic Solutions.
From www.studypool.com
SOLUTION C5 endothermic and exothermic reactions revision guide Endothermic Solutions Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions feel cold. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted. Endothermic Solutions.
From stock.adobe.com
Endothermic Vs Exothermic Reactions Infographic Diagram showing a Endothermic Solutions Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. Because heat is absorbed, endothermic reactions feel cold. An endothermic reaction. Endothermic Solutions.
From www.studypool.com
SOLUTION Exothermic and endothermic reactions Studypool Endothermic Solutions Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry. Endothermic Solutions.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Solutions The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form, although perhaps limiting the. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Read on to learn. Endothermic Solutions.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Endothermic Solutions The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but. Endothermic Solutions.
From question.pandai.org
Endothermic and exothermic reactions Endothermic Solutions Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form, although perhaps limiting the. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but. Endothermic Solutions.
From slideplayer.com
Chapter 13 PROPERTIES OF SOLUTIONS ppt download Endothermic Solutions Because heat is absorbed, endothermic reactions feel cold. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical. Endothermic Solutions.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Solutions Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form, although perhaps limiting. Endothermic Solutions.
From www.slideserve.com
PPT Elements, Mixtures, Compounds and Solutions PowerPoint Endothermic Solutions For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless. Because heat is absorbed, endothermic reactions feel cold. Even if the. Endothermic Solutions.
From www.slideserve.com
PPT Solutions and Their Properties PowerPoint Presentation, free Endothermic Solutions Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless. Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form, although perhaps limiting the. Because heat is absorbed, endothermic reactions feel cold. Read on. Endothermic Solutions.
From online-learning-college.com
Exothermic and endothermic reactions Key differences Endothermic Solutions The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. Endothermic Solutions.
From www.studypool.com
SOLUTION Chemistry exothermic endothermic reactions Studypool Endothermic Solutions Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. Because heat is absorbed, endothermic reactions feel cold. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The enthalpy change of solution refers to the amount of heat that. Endothermic Solutions.
From www.slideserve.com
PPT Heat from Reactions PowerPoint Presentation, free download ID Endothermic Solutions The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form, although perhaps limiting the. Read on to learn. Endothermic Solutions.
From www.slideserve.com
PPT Chapter 7 Solutions and Colloids PowerPoint Presentation, free Endothermic Solutions For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form, although perhaps limiting the. Because heat is absorbed, endothermic reactions feel cold. An endothermic reaction is a chemical reaction that absorbs thermal energy. Endothermic Solutions.
From schempal.com
Understanding the Energy Level Diagram of an Endothermic Reaction Endothermic Solutions Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Even if the energetics are slightly endothermic, the entropy effect can still. Endothermic Solutions.
From www.clutchprep.com
Endothermic & Exothermic Reactions Chemistry Video Clutch Prep Endothermic Solutions For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The enthalpy change of solution refers. Endothermic Solutions.
From www.numerade.com
Is the solution process exothermic (energy released, temperature Endothermic Solutions Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. Because heat is absorbed, endothermic reactions feel cold. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical. Endothermic Solutions.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Solutions The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but. Endothermic Solutions.
From slideplayer.com
The Molecular Nature of Matter and Change ppt download Endothermic Solutions An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Endothermic dissolutions such as this one require a greater energy input to. Endothermic Solutions.
From chemwiki.ucdavis.edu
Full Chapter 13 Properties of Solutions Chemwiki Endothermic Solutions Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. An endothermic reaction is a chemical reaction that absorbs thermal energy. Endothermic Solutions.
From www.flexiprep.com
NCERT Class X Science Solutions Chapter 1 Chemical Reactions and Endothermic Solutions Because heat is absorbed, endothermic reactions feel cold. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless. Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form, although perhaps limiting the. Examples of. Endothermic Solutions.
From www.youtube.com
Interpreting Exothermic and Endothermic Reaction Graphs A Simple Endothermic Solutions Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form, although perhaps limiting the. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions feel cold. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be. Endothermic Solutions.
From www.slideserve.com
PPT Unit 12 Properties of Solutions PowerPoint Presentation, free Endothermic Solutions The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless. Because heat is absorbed, endothermic reactions feel cold. For an. Endothermic Solutions.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Solutions The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form, although perhaps limiting the. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Examples of endothermic reactions. Endothermic Solutions.
From www.youtube.com
Endothermic and exothermic reactions. Enthalpy YouTube Endothermic Solutions The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and. Endothermic Solutions.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Solutions Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless. Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form,. Endothermic Solutions.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Solutions Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when. Endothermic Solutions.
From printablezonedefuse.z13.web.core.windows.net
Endothermic And Exothermic Reactions Worksheet Endothermic Solutions For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form, although perhaps limiting. Endothermic Solutions.
From www.slideserve.com
PPT Energy in Chemical Changes PowerPoint Presentation, free download Endothermic Solutions Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions feel cold. Read on to learn. Endothermic Solutions.
From www.studypool.com
SOLUTION Exothermic and endothermic processes Studypool Endothermic Solutions The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless. An endothermic reaction is a chemical reaction that absorbs thermal. Endothermic Solutions.
From www.slideserve.com
PPT Chapter 11. Solutions and Their Properties PowerPoint Endothermic Solutions An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. The enthalpy change of solution refers. Endothermic Solutions.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Solutions Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless. Even if the energetics are slightly endothermic, the entropy effect can still allow the solution to form, although perhaps limiting the. The enthalpy change of solution refers to the amount of. Endothermic Solutions.
From www.slideserve.com
PPT Types of mixtures PowerPoint Presentation, free download ID4695980 Endothermic Solutions Because heat is absorbed, endothermic reactions feel cold. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry. Endothermic Solutions.
From www.studypool.com
SOLUTION endothermic and exothermic and energy diagrams Studypool Endothermic Solutions An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Read on to learn about how to distinguish endothermic and exothermic reactions, connect them to other chemistry concepts, and see. For an endothermic chemical reaction to proceed, the reactants must absorb energy. Endothermic Solutions.