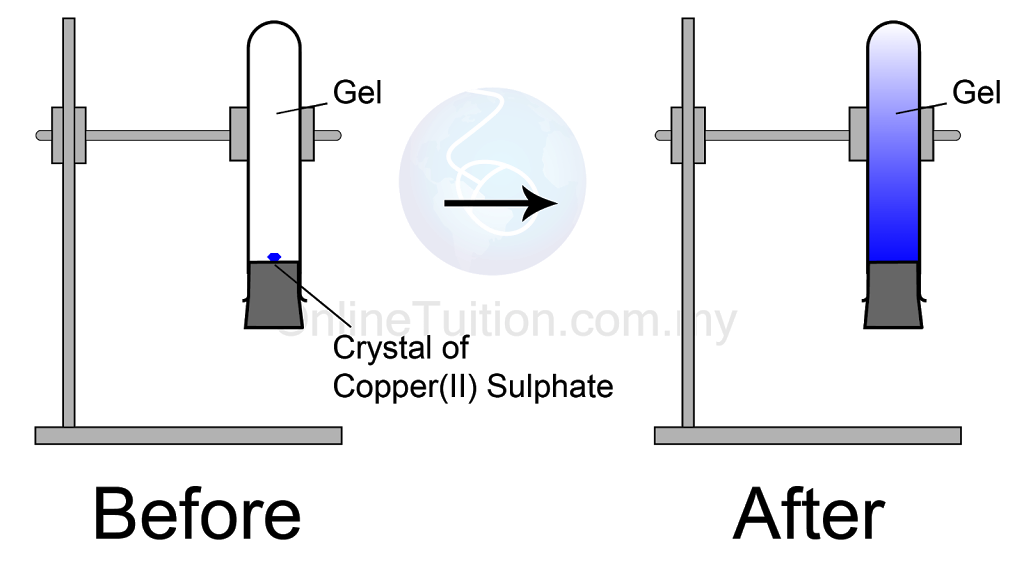
Diffusion Of Solids Liquids . Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. (view the animation below to compare. Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the viscosity of the specific liquid into which the material is diffusing. How can the rate of diffusion. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Since diffusion equalizes the concentration of the. Diffusion is a physical process that refers to the net movement of molecules from a region of high concentration to one of lower. Why is it an important part of processing? Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. Diffusion happens on its own when the particles spread out from an area. Includes kit list and safety instructions.
from spmchemistry.blog.onlinetuition.com.my
At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. Why is it an important part of processing? Includes kit list and safety instructions. Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the viscosity of the specific liquid into which the material is diffusing. Diffusion is a physical process that refers to the net movement of molecules from a region of high concentration to one of lower. (view the animation below to compare. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. How can the rate of diffusion. Diffusion happens on its own when the particles spread out from an area.
Diffusion in Solid SPM Chemistry
Diffusion Of Solids Liquids The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. Why is it an important part of processing? Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the viscosity of the specific liquid into which the material is diffusing. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. Includes kit list and safety instructions. How can the rate of diffusion. Since diffusion equalizes the concentration of the. Diffusion happens on its own when the particles spread out from an area. (view the animation below to compare. Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. Diffusion is a physical process that refers to the net movement of molecules from a region of high concentration to one of lower. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion.
From www.youtube.com
Diffusion of Solids in Liquid Chemistry Investigatory Projects Diffusion Of Solids Liquids At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Since diffusion equalizes the concentration of the. Diffusion happens on its own when the particles spread out from an area. Since the movement of particles is associated with collisions, the path of a single particle is a. Diffusion Of Solids Liquids.
From spmchemistry.blog.onlinetuition.com.my
Diffusion in Liquid SPM Chemistry Diffusion Of Solids Liquids Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Why is it an important part of processing? The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic. Diffusion Of Solids Liquids.
From www.slideserve.com
PPT Behavior of Liquids and Gases Diffusion and Pressure PowerPoint Diffusion Of Solids Liquids Since diffusion equalizes the concentration of the. Diffusion happens on its own when the particles spread out from an area. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. (view the animation. Diffusion Of Solids Liquids.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Diffusion Of Solids Liquids Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. (view the animation below to compare. Diffusion in liquids is proportional to temperature, as it is in gases, as well as to. Diffusion Of Solids Liquids.
From present5.com
The Transport of Materials Across the Cell membrane Diffusion Of Solids Liquids Diffusion happens on its own when the particles spread out from an area. Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the viscosity of the specific liquid into which the material is diffusing. (view the animation below to compare. Since the movement of particles is associated with collisions, the path of a. Diffusion Of Solids Liquids.
From www.studypool.com
SOLUTION Diffusion in solids liquids and gases Studypool Diffusion Of Solids Liquids (view the animation below to compare. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Since diffusion equalizes the concentration of the. Why is it an important part of processing? Diffusion is how smells spread out through the air and how concentrated liquids spread out when. Diffusion Of Solids Liquids.
From thescienceteacher.co.uk
Diffusion teaching resources the science teacher Diffusion Of Solids Liquids Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the viscosity of the specific liquid into which the material is diffusing. Why is it an important part of processing? (view the animation below to compare. Since the movement of particles is associated with collisions, the path of a single particle is a zigzag. Diffusion Of Solids Liquids.
From www.slideshare.net
solids, liquids and gases Diffusion Of Solids Liquids Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the viscosity of the specific liquid into which the material is diffusing. Includes kit list and safety instructions. Since diffusion equalizes the concentration of the.. Diffusion Of Solids Liquids.
From www.sciencefacts.net
Diffusion Definition and How Does it Occur (with Diagram) Diffusion Of Solids Liquids The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. How can the rate of diffusion. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Diffusion is a physical process that refers to the net movement of molecules from. Diffusion Of Solids Liquids.
From www.visionlearning.com
Diffusion I Chemistry Visionlearning Diffusion Of Solids Liquids Diffusion happens on its own when the particles spread out from an area. Includes kit list and safety instructions. Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. Since diffusion equalizes the concentration of the. Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the viscosity. Diffusion Of Solids Liquids.
From www.youtube.com
Diffusion class 9 Diffusion in solids Diffusion in liquids Diffusion Of Solids Liquids Why is it an important part of processing? Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. Diffusion happens on its own when the particles spread out from an area. How can the rate of diffusion. Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the. Diffusion Of Solids Liquids.
From www.teachoo.com
Diffusion in Solids, Liquids and Gases with Examples Teachoo Diffusion Of Solids Liquids Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. Diffusion happens on its own when the particles spread out from an area. Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. At any temperature different from absolute zero all atoms, irrespective of their. Diffusion Of Solids Liquids.
From www.scribd.com
To Study The Diffusion of Solids in Liquids PDF Particle Diffusion Diffusion Of Solids Liquids At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. How can the rate of diffusion. Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the viscosity of the specific liquid into which the material is diffusing. Includes kit list. Diffusion Of Solids Liquids.
From eduinput.com
Why is Diffusion Faster in Liquids than Solids? Diffusion Of Solids Liquids Diffusion happens on its own when the particles spread out from an area. Why is it an important part of processing? The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. How can the rate of diffusion. (view the animation below to compare. Since diffusion equalizes the concentration of. Diffusion Of Solids Liquids.
From www.studocu.com
Icbse project TOPIC Study of Diffusion of solids in Liquids BY Diffusion Of Solids Liquids Diffusion is a physical process that refers to the net movement of molecules from a region of high concentration to one of lower. Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. Why is it an important part of processing? Diffusion is how smells spread out through the air and how concentrated liquids. Diffusion Of Solids Liquids.
From www.snexplores.org
Explainer What are the different states of matter? Diffusion Of Solids Liquids Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. Diffusion happens on its own when the particles spread out from an area. Why is it an important part of processing? The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the. Diffusion Of Solids Liquids.
From byjus.com
Explain the diffusion in liquids with two examples. Diffusion Of Solids Liquids Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. (view the animation below to compare. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. How can the rate of diffusion. At any temperature different from absolute zero all. Diffusion Of Solids Liquids.
From www.youtube.com
Diffusion in Solid Chemistry YouTube Diffusion Of Solids Liquids Since diffusion equalizes the concentration of the. Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. (view the animation below to compare. Diffusion is a physical process that refers to the net movement of molecules from a region of high concentration to one of lower. At any temperature different from. Diffusion Of Solids Liquids.
From www.visionlearning.com
Visionlearning Chemistry Diffusion I Diffusion Of Solids Liquids How can the rate of diffusion. Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the. Diffusion Of Solids Liquids.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6716640 Diffusion Of Solids Liquids Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. Since diffusion equalizes the concentration of the. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous,. Diffusion Of Solids Liquids.
From byjus.com
What is diffusion? Explain about diffusion in solid , liquid and gas Diffusion Of Solids Liquids Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the viscosity of the specific liquid into which the material is diffusing. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. Since the movement of particles is associated with collisions, the path. Diffusion Of Solids Liquids.
From www.teachoo.com
Diffusion in Solids, Liquids and Gases with Examples Teachoo Diffusion Of Solids Liquids Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the viscosity of the. Diffusion Of Solids Liquids.
From www.shutterstock.com
31 Diffusion solids liquids Images, Stock Photos & Vectors Shutterstock Diffusion Of Solids Liquids At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. Diffusion happens on its own when the particles spread out from an area. (view the animation. Diffusion Of Solids Liquids.
From spmchemistry.blog.onlinetuition.com.my
Diffusion in Solid SPM Chemistry Diffusion Of Solids Liquids Since diffusion equalizes the concentration of the. Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. Diffusion is a physical process that refers to the net movement of molecules from a region of high concentration to one of lower. Includes kit list and safety instructions. How can the rate of diffusion. (view the. Diffusion Of Solids Liquids.
From www.slideserve.com
PPT Chapter 12 Intermolecular Attractions and the Properties of Diffusion Of Solids Liquids Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the viscosity of the specific liquid into which the material is diffusing. Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the. Diffusion Of Solids Liquids.
From dxojeueds.blob.core.windows.net
Solids Liquids And Gases Third Grade at Ann Trotter blog Diffusion Of Solids Liquids Diffusion is a physical process that refers to the net movement of molecules from a region of high concentration to one of lower. (view the animation below to compare. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. Why is it an important part of processing? Demonstrate that. Diffusion Of Solids Liquids.
From www.slideserve.com
PPT Water and its relation to Diffusion and Osmosis PowerPoint Diffusion Of Solids Liquids Why is it an important part of processing? At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Since diffusion equalizes the concentration of the. Since the movement. Diffusion Of Solids Liquids.
From www.slideserve.com
PPT Lecture 17 Diffusion PowerPoint Presentation, free download ID Diffusion Of Solids Liquids Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. (view the animation below to compare. Includes kit list and safety instructions. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Diffusion is how smells spread out. Diffusion Of Solids Liquids.
From www.teachoo.com
Diffusion in Solids, Liquids and Gases with Examples Teachoo Diffusion Of Solids Liquids Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the viscosity of the specific liquid into which the material is diffusing. Why is it an important part of processing? Diffusion is a physical process. Diffusion Of Solids Liquids.
From www.thoughtco.com
A Definition of Diffusion in Chemistry Diffusion Of Solids Liquids Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. Diffusion is a physical process that refers to the net movement of molecules from a region of high concentration to. Diffusion Of Solids Liquids.
From www.slideserve.com
PPT CHAPTER 5 DIFFUSION IN SOLIDS PowerPoint Presentation, free Diffusion Of Solids Liquids Diffusion happens on its own when the particles spread out from an area. How can the rate of diffusion. Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. (view the animation below to compare. Diffusion in liquids is proportional to temperature, as it is in gases, as well as to. Diffusion Of Solids Liquids.
From www.slideserve.com
PPT The Plasma Membrane PowerPoint Presentation, free download ID Diffusion Of Solids Liquids Includes kit list and safety instructions. Diffusion in liquids is proportional to temperature, as it is in gases, as well as to the viscosity of the specific liquid into which the material is diffusing. Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. Diffusion is a physical process that refers to the net. Diffusion Of Solids Liquids.
From www.slideserve.com
PPT Lecture 3a. PowerPoint Presentation, free download ID814388 Diffusion Of Solids Liquids Includes kit list and safety instructions. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. (view the animation below to compare. Diffusion in liquids is proportional to temperature, as. Diffusion Of Solids Liquids.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Diffusion Of Solids Liquids Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water. (view the animation below to compare. The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of. Diffusion Of Solids Liquids.
From www.popoptiq.com
3 Types of Diffusion (Plus Examples for Each) Diffusion Of Solids Liquids The random movement of ‘molecules existing in any state of solid, liquid, or gas’ increases the kinetic energy of the system. Diffusion happens on its own when the particles spread out from an area. Demonstrate that diffusion takes place in liquids in this practical using lead nitrate and potassium iodide. Diffusion in liquids is proportional to temperature, as it is. Diffusion Of Solids Liquids.