Standard Heat Of Formation Vs Standard Heat Of Reaction . A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. The elemental form of each atom is that with the lowest enthalpy in the standard state. An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The main difference between heat of formation and heat of reaction is that heat of formation is the amount of energy either absorbed or released during the.
from brunofuga.adv.br
The main difference between heat of formation and heat of reaction is that heat of formation is the amount of energy either absorbed or released during the. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. The elemental form of each atom is that with the lowest enthalpy in the standard state. An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF
Standard Heat Of Formation Vs Standard Heat Of Reaction An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. The elemental form of each atom is that with the lowest enthalpy in the standard state. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The main difference between heat of formation and heat of reaction is that heat of formation is the amount of energy either absorbed or released during the. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their.
From www.chegg.com
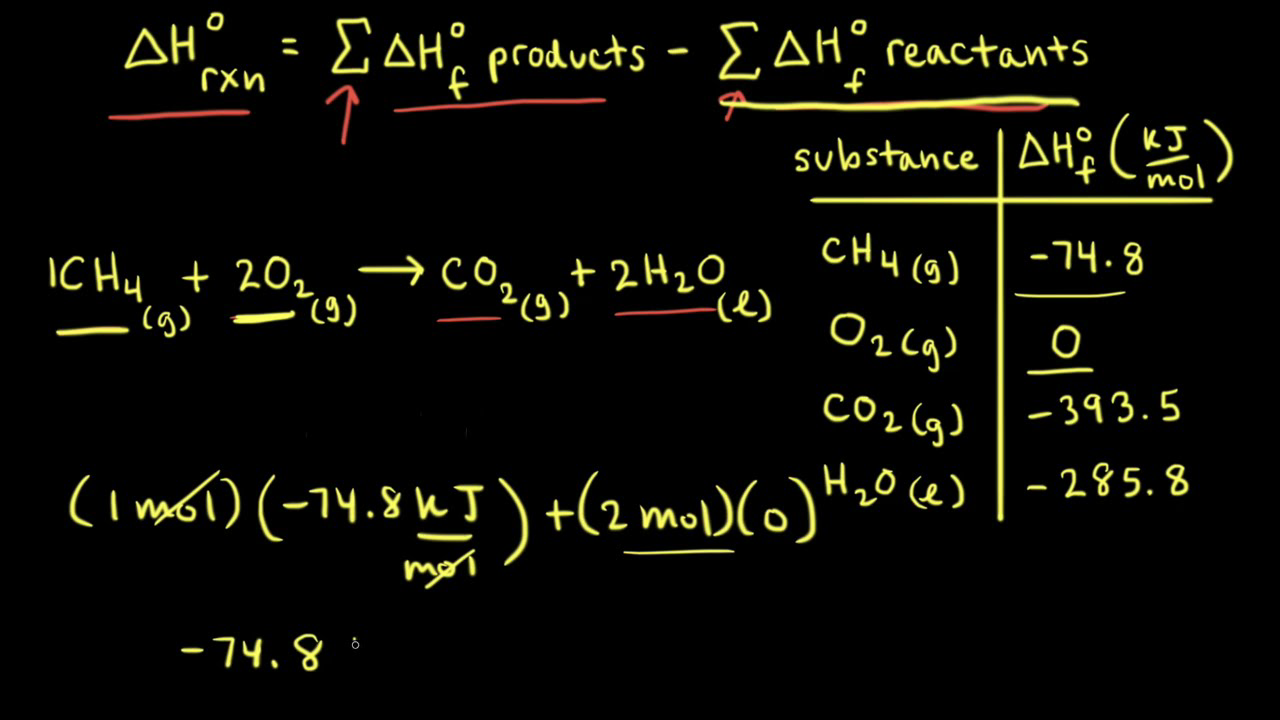
Solved Use the standard enthalpies of formation in the table Standard Heat Of Formation Vs Standard Heat Of Reaction The main difference between heat of formation and heat of reaction is that heat of formation is the amount of energy either absorbed or released during the. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. The standard state for measuring and reporting enthalpies of formation. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation Vs Standard Heat Of Reaction 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. The main difference between heat of formation and heat of reaction is that heat of formation is the. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Heat Of Formation Vs Standard Heat Of Reaction The main difference between heat of formation and heat of reaction is that heat of formation is the amount of energy either absorbed or released during the. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. A standard enthalpy of formation δ h f ° δ. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Heat Of Formation Vs Standard Heat Of Reaction The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.slideserve.com
PPT Chemistry 17.4 PowerPoint Presentation, free download ID2772524 Standard Heat Of Formation Vs Standard Heat Of Reaction The main difference between heat of formation and heat of reaction is that heat of formation is the amount of energy either absorbed or released during the. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation is the enthalpy. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From cevrpozx.blob.core.windows.net
Standard Heat Of Formation Co at Tamara Schultz blog Standard Heat Of Formation Vs Standard Heat Of Reaction A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. The main difference between heat of formation and heat of reaction is that heat of formation is the amount of energy either absorbed or released during the. If we have values for the appropriate. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.youtube.com
Standard Heat of Reaction and Formation YouTube Standard Heat Of Formation Vs Standard Heat Of Reaction 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Heat Of Formation Vs Standard Heat Of Reaction A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5773812 Standard Heat Of Formation Vs Standard Heat Of Reaction The main difference between heat of formation and heat of reaction is that heat of formation is the amount of energy either absorbed or released during the. An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. The standard state for measuring and reporting enthalpies of formation or reaction is 25. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation Vs Standard Heat Of Reaction The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.congress-intercultural.eu
Different Types Of Heat (Enthalpy) Of Reaction Read, 42 OFF Standard Heat Of Formation Vs Standard Heat Of Reaction The main difference between heat of formation and heat of reaction is that heat of formation is the amount of energy either absorbed or released during the. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. A standard enthalpy of formation δ h f ° δ h. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.msjchem.com
Topic 5 Energetics MSJChem Tutorial videos for IB Chemistry Standard Heat Of Formation Vs Standard Heat Of Reaction A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The elemental form of each atom is that. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Heat Of Formation Vs Standard Heat Of Reaction If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From quizzlistreplevies.z13.web.core.windows.net
How To Calculate The Heat Of Formation Standard Heat Of Formation Vs Standard Heat Of Reaction The elemental form of each atom is that with the lowest enthalpy in the standard state. An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Heat Of Formation Vs Standard Heat Of Reaction 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. The main difference between heat of formation and heat of reaction is that heat of formation is the. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.youtube.com
Heat of Reaction (from Heat of Formation) YouTube Standard Heat Of Formation Vs Standard Heat Of Reaction An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. The elemental form of each atom is that with the lowest enthalpy in the standard state. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. If we have. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.vrogue.co
Standard Enthalpy Of Reaction Units Slide Share vrogue.co Standard Heat Of Formation Vs Standard Heat Of Reaction 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The elemental form of each atom is that with the lowest enthalpy in the standard state. An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. A standard. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From duanerafanan.blogspot.com
DUANE HESS'S LAW Standard Heat Of Formation Vs Standard Heat Of Reaction A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. The elemental form of each atom is that with the lowest. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.chegg.com
Solved The standard enthalpy (or heat) of reaction, delta H, Standard Heat Of Formation Vs Standard Heat Of Reaction An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. The elemental form of each atom is that with the lowest enthalpy in the standard state. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Heat Of Formation Vs Standard Heat Of Reaction A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The main difference between heat of formation and heat of reaction is that heat of formation is the amount of energy either absorbed or released during the. If we have values for the appropriate. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From truyenhinhcapsongthu.net
Difference Between Heat Of Formation And Heat Of Reaction Standard Heat Of Formation Vs Standard Heat Of Reaction A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. An enthalpy change that occurs specifically under standard conditions is called. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Heat Of Formation Vs Standard Heat Of Reaction A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. A standard enthalpy of formation δ h f ° δ h f. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.youtube.com
Standard Heat of Formation & Calculating Standard Heat of Reaction Standard Heat Of Formation Vs Standard Heat Of Reaction The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.numerade.com
SOLVED 'The following problems deal with standard enthalpy of Standard Heat Of Formation Vs Standard Heat Of Reaction A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. Standard enthalpy of formation is the enthalpy change when one mole of any compound is. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.slideserve.com
PPT Advanced Thermodynamics Note 3 Heat Effects PowerPoint Standard Heat Of Formation Vs Standard Heat Of Reaction A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID314871 Standard Heat Of Formation Vs Standard Heat Of Reaction The main difference between heat of formation and heat of reaction is that heat of formation is the amount of energy either absorbed or released during the. The elemental form of each atom is that with the lowest enthalpy in the standard state. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.youtube.com
Std Heat of Formation versus Bond Energy YouTube Standard Heat Of Formation Vs Standard Heat Of Reaction The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. The main difference between heat of formation and heat of reaction is that heat of. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Formation Vs Standard Heat Of Reaction A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Heat Of Formation Vs Standard Heat Of Reaction An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. The elemental form of each atom is that with the lowest enthalpy in the standard state. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. If we. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Heat Of Formation Vs Standard Heat Of Reaction A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. The standard state for measuring and reporting enthalpies of formation or reaction is 25 o. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.coursehero.com
[Solved] 1. All of the following compounds have a standard heat of Standard Heat Of Formation Vs Standard Heat Of Reaction If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. The main difference between heat of formation and heat of reaction is that heat of formation is the amount of energy either absorbed or released during the. A standard enthalpy of formation $δh°_f$ is an enthalpy change. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From byjus.com
Standard heat of formation of ammonia is x kJ/mol.The heat of reaction Standard Heat Of Formation Vs Standard Heat Of Reaction The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for any reaction, which we will. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.vrogue.co
Standard Enthalpy Of Formation And Standard Free Ener vrogue.co Standard Heat Of Formation Vs Standard Heat Of Reaction The standard state for measuring and reporting enthalpies of formation or reaction is 25 o c and 1 atm. The main difference between heat of formation and heat of reaction is that heat of formation is the amount of energy either absorbed or released during the. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.numerade.com
SOLVED Formation Reactions Review Constants Periodic Table The Standard Heat Of Formation Vs Standard Heat Of Reaction A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The elemental form of each atom is that with the lowest enthalpy in the standard state. If we have values for the appropriate standard enthalpies of formation, we can determine the enthalpy change for. Standard Heat Of Formation Vs Standard Heat Of Reaction.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Heat Of Formation Vs Standard Heat Of Reaction An enthalpy change that occurs specifically under standard conditions is called the standard enthalpy (or heat) of reaction and. Standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent elements in their. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of. Standard Heat Of Formation Vs Standard Heat Of Reaction.