Bromine Oxygen . Nascent oxygen is a potent oxidizer, capable of. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. These elements are called halogens, from greek roots translating to salt formers. the halogens react with oxygen, but many of the resulting compounds are unstable, lasting for only moments at a time. While some uses of bromine have declined because the products made from it are no longer needed, others have been discouraged because. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. The elements in group 17 include fluorine, chlorine, bromine, and iodine. They range in structure from x Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2 and bromine trioxide bro 3 are studied in ab initio correlated. Compare elements on more than 90 properties. Compare bromine and oxygen on the basis of their properties, attributes and periodic table facts. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate.
from www.numerade.com
Compare bromine and oxygen on the basis of their properties, attributes and periodic table facts. Nascent oxygen is a potent oxidizer, capable of. These elements are called halogens, from greek roots translating to salt formers. the halogens react with oxygen, but many of the resulting compounds are unstable, lasting for only moments at a time. The elements in group 17 include fluorine, chlorine, bromine, and iodine. Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2 and bromine trioxide bro 3 are studied in ab initio correlated. Compare elements on more than 90 properties. They range in structure from x The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds.
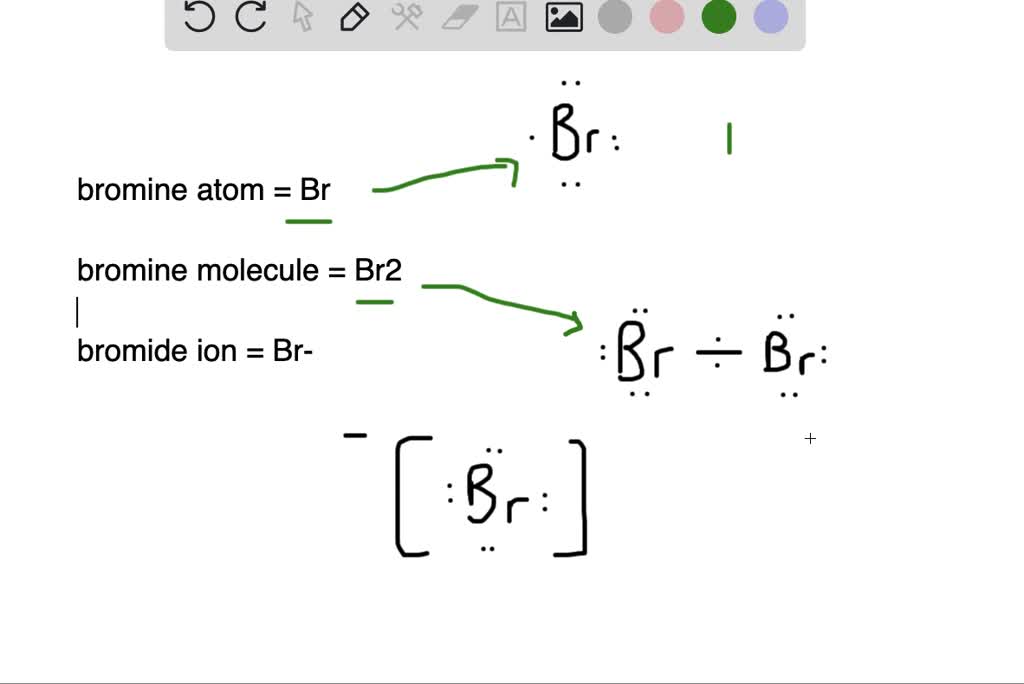
What is the difference between (a) a bromine atom, (b) a bromine
Bromine Oxygen While some uses of bromine have declined because the products made from it are no longer needed, others have been discouraged because. These elements are called halogens, from greek roots translating to salt formers. the halogens react with oxygen, but many of the resulting compounds are unstable, lasting for only moments at a time. Compare bromine and oxygen on the basis of their properties, attributes and periodic table facts. They range in structure from x The elements in group 17 include fluorine, chlorine, bromine, and iodine. Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2 and bromine trioxide bro 3 are studied in ab initio correlated. Compare elements on more than 90 properties. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Nascent oxygen is a potent oxidizer, capable of. While some uses of bromine have declined because the products made from it are no longer needed, others have been discouraged because. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Oxygen Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2 and bromine trioxide bro 3 are studied in ab initio correlated. These elements are called halogens, from greek roots translating to salt formers. the halogens react with oxygen, but many of the. Bromine Oxygen.
From courses.lumenlearning.com
Chemical Equilibria Chemistry for Majors Bromine Oxygen Compare bromine and oxygen on the basis of their properties, attributes and periodic table facts. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. The elements in group. Bromine Oxygen.
From www.researchgate.net
Schematic drawing of the three typical stages in the SPERISE process Bromine Oxygen Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Compare bromine and oxygen on the basis of their properties, attributes and periodic table facts. Nascent oxygen is a potent oxidizer, capable of. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate.. Bromine Oxygen.
From www.numerade.com
SOLVED Consider the bromite BrO2 anion What is the central atom Bromine Oxygen Compare elements on more than 90 properties. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2 and bromine trioxide bro 3 are studied in ab. Bromine Oxygen.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Oxygen Compare elements on more than 90 properties. Compare bromine and oxygen on the basis of their properties, attributes and periodic table facts. Nascent oxygen is a potent oxidizer, capable of. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. These elements are called halogens, from greek roots translating to salt formers. the halogens. Bromine Oxygen.
From www.vectorstock.com
Br2 bromine molecule Royalty Free Vector Image Bromine Oxygen While some uses of bromine have declined because the products made from it are no longer needed, others have been discouraged because. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2 and bromine trioxide bro 3 are studied in ab. Bromine Oxygen.
From www.youtube.com
How to Draw the Lewis Dot Structure for Br2 Diatomic Bromine YouTube Bromine Oxygen While some uses of bromine have declined because the products made from it are no longer needed, others have been discouraged because. Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2 and bromine trioxide bro 3 are studied in ab initio correlated. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent. Bromine Oxygen.
From www.britannica.com
bromine Properties, Uses, & Facts Britannica Bromine Oxygen The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2 and bromine trioxide bro 3 are studied in ab initio correlated. In the aqueous bulk, oxidation of bromide by ozone involves a. Bromine Oxygen.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Oxygen Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2 and bromine trioxide bro 3 are studied in ab initio correlated. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Nascent oxygen is a potent oxidizer, capable of. The elements in group 17 include fluorine, chlorine, bromine, and iodine. These. Bromine Oxygen.
From www.amazon.fr
EDG by AQUALUX Brome Choc Pastilles 20g 1kg Activateur de Brome Bromine Oxygen In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Nascent oxygen is a potent oxidizer, capable of. The elements in group 17 include fluorine, chlorine, bromine, and iodine.. Bromine Oxygen.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Bromine Oxygen Nascent oxygen is a potent oxidizer, capable of. Compare elements on more than 90 properties. They range in structure from x In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. While some uses of bromine have declined because the products made from it are no longer needed, others have been discouraged because. Several. Bromine Oxygen.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Oxygen Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Compare elements on more than 90 properties. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers. Bromine Oxygen.
From www.filtres-spa.com
Utiliser brome et oxygène actif estce compatible Bromine Oxygen The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. These elements are called halogens, from greek roots translating to salt formers. the halogens react with oxygen, but many of the resulting compounds are unstable, lasting for only moments at a time. Compare elements on. Bromine Oxygen.
From fphoto.photoshelter.com
science chemistry reaction bromine absorption charcoal Fundamental Bromine Oxygen Nascent oxygen is a potent oxidizer, capable of. Compare elements on more than 90 properties. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. While some uses of bromine have declined because the products made from it are no longer needed, others have been. Bromine Oxygen.
From stock.adobe.com
Vecteur Stock Diatomic molecules diagram shows elements that exist as Bromine Oxygen These elements are called halogens, from greek roots translating to salt formers. the halogens react with oxygen, but many of the resulting compounds are unstable, lasting for only moments at a time. They range in structure from x In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Due to its potent oxidatizing action,. Bromine Oxygen.
From www.masterorganicchemistry.com
Bromination of alkenes with Br2 to give dibromides Master Organic Bromine Oxygen Compare bromine and oxygen on the basis of their properties, attributes and periodic table facts. They range in structure from x In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen. Bromine Oxygen.
From myloview.com
Nabro3 sodium bromate molecule. simple molecular formula consisting Bromine Oxygen While some uses of bromine have declined because the products made from it are no longer needed, others have been discouraged because. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. These elements. Bromine Oxygen.
From www.livescience.com
Facts About Bromine Live Science Bromine Oxygen While some uses of bromine have declined because the products made from it are no longer needed, others have been discouraged because. Compare elements on more than 90 properties. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2 and bromine trioxide. Bromine Oxygen.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Bromine Oxygen Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. Several isomers of bromine dioxide bro 2, dibromine. Bromine Oxygen.
From www.dreamstime.com
Bromine Chemical Element Periodic Table Symbol 3d Render Stock Bromine Oxygen In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. The elements in group 17 include fluorine, chlorine, bromine, and iodine. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. Compare bromine and oxygen on the basis of their properties, attributes and. Bromine Oxygen.
From www.deviantart.com
sodium bromine oxygen by SparkyKitsune on DeviantArt Bromine Oxygen These elements are called halogens, from greek roots translating to salt formers. the halogens react with oxygen, but many of the resulting compounds are unstable, lasting for only moments at a time. Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2 and bromine trioxide bro 3 are studied in ab initio correlated. While some uses of. Bromine Oxygen.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Oxygen Nascent oxygen is a potent oxidizer, capable of. They range in structure from x Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. The elements in group 17 include fluorine, chlorine, bromine, and iodine. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent. Bromine Oxygen.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy Bromine Oxygen Compare elements on more than 90 properties. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. They range in structure from x Compare bromine and oxygen on the basis of their properties, attributes and periodic table facts. The first ionization energy of bromine is high, and compounds containing. Bromine Oxygen.
From ifunny.co
Scientist Do you know what sodium hydrogen, bromine, and oxygen form Bromine Oxygen They range in structure from x Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. These elements are called halogens, from greek roots translating to salt formers. the halogens react with oxygen, but many of the resulting compounds are unstable, lasting for only moments at a time. While. Bromine Oxygen.
From www.alamy.com
Bromine. Bromum. Halogens. Chemical Element of Mendeleev's Periodic Bromine Oxygen Compare elements on more than 90 properties. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2 and bromine trioxide bro 3 are studied in ab initio correlated. These elements are called halogens, from greek roots translating to salt formers. the halogens. Bromine Oxygen.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Oxygen Compare elements on more than 90 properties. They range in structure from x These elements are called halogens, from greek roots translating to salt formers. the halogens react with oxygen, but many of the resulting compounds are unstable, lasting for only moments at a time. Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2 and bromine. Bromine Oxygen.
From www.chegg.com
Solved O CHEMICAL BONDING Predicting deviations from ideal Bromine Oxygen Compare bromine and oxygen on the basis of their properties, attributes and periodic table facts. The elements in group 17 include fluorine, chlorine, bromine, and iodine. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Several isomers of bromine dioxide bro 2, dibromine dioxide. Bromine Oxygen.
From narodnatribuna.info
Bromine Atomic Mass Bromine Oxygen These elements are called halogens, from greek roots translating to salt formers. the halogens react with oxygen, but many of the resulting compounds are unstable, lasting for only moments at a time. Nascent oxygen is a potent oxidizer, capable of. Compare bromine and oxygen on the basis of their properties, attributes and periodic table facts. The first ionization energy of. Bromine Oxygen.
From www.researchgate.net
Hydrogen bonds of two independent C5N2H14 2+ cations. Bromine olive Bromine Oxygen Nascent oxygen is a potent oxidizer, capable of. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. These elements are called halogens, from greek roots translating to salt formers.. Bromine Oxygen.
From periodictable.me
2000pxElectron_configuration_bromine.svg Dynamic Periodic Table of Bromine Oxygen Compare bromine and oxygen on the basis of their properties, attributes and periodic table facts. They range in structure from x The elements in group 17 include fluorine, chlorine, bromine, and iodine. While some uses of bromine have declined because the products made from it are no longer needed, others have been discouraged because. Due to its potent oxidatizing action,. Bromine Oxygen.
From stock.adobe.com
Vecteur Stock NaBrO3 Sodium Bromate molecule. Simple molecular formula Bromine Oxygen While some uses of bromine have declined because the products made from it are no longer needed, others have been discouraged because. Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2 and bromine trioxide bro 3 are studied in ab initio correlated. These elements are called halogens, from greek roots translating to salt formers. the halogens. Bromine Oxygen.
From www.researchgate.net
The short bromineoxygen close contacts connecting the hydrogen bonded Bromine Oxygen The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. The elements in group 17 include fluorine, chlorine, bromine, and iodine. Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. While some uses. Bromine Oxygen.
From gradegorilla.com
Gradegorilla Chemistry Bromine Oxygen Due to its potent oxidatizing action, bromine liberates nascent oxygen or oxygen free radicals from the water present in mucous membranes. They range in structure from x These elements are called halogens, from greek roots translating to salt formers. the halogens react with oxygen, but many of the resulting compounds are unstable, lasting for only moments at a time. In. Bromine Oxygen.
From images-of-elements.com
Chemical Elements Bromine Bromine Oxygen The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. While some uses of bromine have declined because the products made from it are no longer needed, others have been discouraged because. The elements in group 17 include fluorine, chlorine, bromine, and iodine. Compounds with. Bromine Oxygen.
From pubs.acs.org
Bromine Radical (Br• and Br2•) Reactivity with Dissolved Organic Bromine Oxygen The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Compare elements on more than 90 properties. Compare bromine and oxygen on the basis of their properties, attributes and periodic table facts. Several isomers of bromine dioxide bro 2, dibromine dioxide br 2 o 2. Bromine Oxygen.