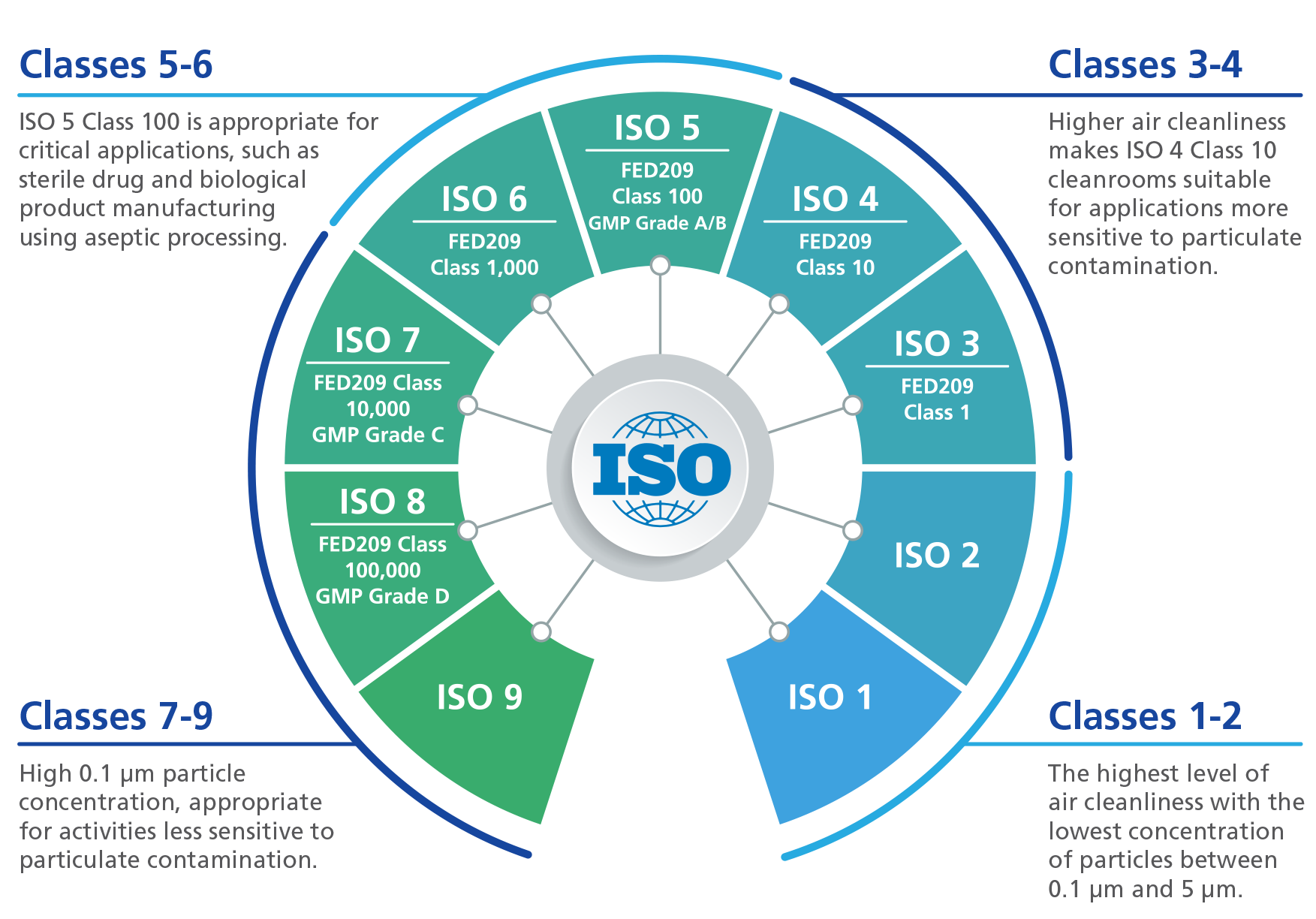
Clean Room Classification As Per Who . This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. It was agreed that the guidelines be amended to comprise two documents: The who expert committee on specifi cations for. Who good practices for pharmaceutical microbiology laboratories. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. One that would consist of guidelines containing recommendations for. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu.
from www.phchd.com
It was agreed that the guidelines be amended to comprise two documents: Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. The who expert committee on specifi cations for. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. Who good practices for pharmaceutical microbiology laboratories. This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. One that would consist of guidelines containing recommendations for.
ISO and GMP Cleanroom Standards PHCbi
Clean Room Classification As Per Who Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. It was agreed that the guidelines be amended to comprise two documents: The who expert committee on specifi cations for. One that would consist of guidelines containing recommendations for. This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. Who good practices for pharmaceutical microbiology laboratories.
From www.angstromcleanroomsupply.com
Cleanroom Classification Angstrom Supply Clean Room Classification As Per Who It was agreed that the guidelines be amended to comprise two documents: Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. Who good practices for pharmaceutical microbiology laboratories. One that would consist of guidelines containing recommendations for. The who expert committee on specifi cations for. For. Clean Room Classification As Per Who.
From pharmastate.academy
Cleanroom Classifications, Classes and ISO Standards Clean Room Classification As Per Who The who expert committee on specifi cations for. This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. One that would consist of guidelines containing recommendations for. It was agreed that the guidelines be amended to comprise two documents: For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and. Clean Room Classification As Per Who.
From www.phchd.com
ISO and GMP Cleanroom Standards PHCbi Clean Room Classification As Per Who This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. One that would consist of guidelines containing recommendations for. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. Who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi. Clean Room Classification As Per Who.
From angstromtechnology.com
Cleanroom Classifications & Standards Angstrom Technology Clean Room Classification As Per Who Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. The who expert committee on specifi cations for. Who good practices for pharmaceutical microbiology laboratories. This document was prepared. Clean Room Classification As Per Who.
From www.presentationeze.com
Cleanroom Classification ISO 14644PresentationEZE Clean Room Classification As Per Who It was agreed that the guidelines be amended to comprise two documents: Who good practices for pharmaceutical microbiology laboratories. This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. The who expert. Clean Room Classification As Per Who.
From ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Clean Room Classification As Per Who One that would consist of guidelines containing recommendations for. This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. Who good practices for pharmaceutical microbiology laboratories. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. For initial classification, or classification following. Clean Room Classification As Per Who.
From pharmacyscope.com
Clean Area Classification Pharmacy Scope Clean Room Classification As Per Who This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. One that would consist of guidelines containing recommendations for. It was agreed that the guidelines be amended to comprise two documents: The who expert committee on specifi cations for. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and. Clean Room Classification As Per Who.
From www.setra.com
What is ISO 8 Cleanroom Classification Clean Room Classification As Per Who Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. It was agreed that the guidelines be amended to comprise two documents: For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. This document was prepared by technical committee. Clean Room Classification As Per Who.
From www.terrauniversal.com
ISO 14644 Types of Cleanrooms & Components Comparison & Features Guide Clean Room Classification As Per Who It was agreed that the guidelines be amended to comprise two documents: The who expert committee on specifi cations for. This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. For initial. Clean Room Classification As Per Who.
From www.iqsdirectory.com
Cleanroom What is it? ISO Standards and Classifications, Design, Types Clean Room Classification As Per Who This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. Who good practices for pharmaceutical microbiology laboratories. It was agreed that the guidelines be amended to comprise two documents: Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. One that would. Clean Room Classification As Per Who.
From moduluscleanrooms.com
Cleanroom ISO Classifications ISO Cleanroom Standards Clean Room Classification As Per Who One that would consist of guidelines containing recommendations for. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements. Clean Room Classification As Per Who.
From www.presentationeze.com
Cleanroom Classification ISO 14644PresentationEZE Clean Room Classification As Per Who This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. Who good practices for pharmaceutical microbiology laboratories. One that would consist of guidelines containing recommendations for. It was agreed that the guidelines be amended to comprise two documents: The who expert committee on specifi cations for. For initial classification, or classification following significant change, it. Clean Room Classification As Per Who.
From www.testotis.com
Worth knowing from the area of cleanroom qualification Clean Room Classification As Per Who It was agreed that the guidelines be amended to comprise two documents: This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. Who good practices for pharmaceutical microbiology laboratories. One that would consist of guidelines containing recommendations. Clean Room Classification As Per Who.
From ansaripharmaeducation.blogspot.com
CLEAN ROOM CLASSIFICATION CLEAN AREA CLASSIFICATION IN MICROBIOLOGY Clean Room Classification As Per Who This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. The who expert committee on specifi cations for. One that would consist of guidelines containing recommendations for. It was agreed that the guidelines be amended to comprise two documents: Who good practices for pharmaceutical microbiology laboratories. For initial classification, or classification following significant change, it. Clean Room Classification As Per Who.
From schillingengineering.de
Cleanroom classes according to ISO 146441 and GMP Clean Room Classification As Per Who It was agreed that the guidelines be amended to comprise two documents: Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. The who expert committee on specifi cations for. One that. Clean Room Classification As Per Who.
From cmmonline.com
Basic Cleanroom Requirements and Classifications Clean Room Classification As Per Who It was agreed that the guidelines be amended to comprise two documents: This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. Who good practices for pharmaceutical microbiology laboratories. One that would consist of guidelines containing recommendations for. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between. Clean Room Classification As Per Who.
From www.iqsdirectory.com
Cleanroom What is it? ISO Standards and Classifications, Design, Types Clean Room Classification As Per Who For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. The who expert committee on specifi cations for. This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. It was agreed that the guidelines be amended to comprise two documents: One that would consist of guidelines containing. Clean Room Classification As Per Who.
From carolinaflow.com
Cleanroom Classifications Carolina Components Group Clean Room Classification As Per Who For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. It was agreed that. Clean Room Classification As Per Who.
From www.portafab.com
Cleanroom Classification & ISO Standards Cleanroom Considerations Clean Room Classification As Per Who It was agreed that the guidelines be amended to comprise two documents: Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. One that would consist of guidelines containing recommendations for. Who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi cations for. This. Clean Room Classification As Per Who.
From www.presentationeze.com
Cleanroom Classification ISO 14644PresentationEZE Clean Room Classification As Per Who For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. The who expert committee on specifi cations for. One that would consist of guidelines containing recommendations for. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. Who good. Clean Room Classification As Per Who.
From ansaripharmaeducation.blogspot.com
CLEAN ROOM CLASSIFICATION CLEAN AREA CLASSIFICATION IN MICROBIOLOGY Clean Room Classification As Per Who It was agreed that the guidelines be amended to comprise two documents: One that would consist of guidelines containing recommendations for. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. The who expert committee on specifi cations for. For initial classification, or classification following significant change,. Clean Room Classification As Per Who.
From gmpinsiders.com
GMP Cleanroom Classifications Understand Class A, B, C And D Clean Room Classification As Per Who The who expert committee on specifi cations for. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. It was agreed that the guidelines be amended to comprise two documents: For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5. Clean Room Classification As Per Who.
From www.aroundlabnews.com
AROUND LAB NEWS / IT » Understanding Cleanroom Classifications Clean Room Classification As Per Who This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. It was agreed that the guidelines be amended to comprise two documents: For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. The who expert committee on specifi cations for. Who good practices for pharmaceutical microbiology laboratories.. Clean Room Classification As Per Who.
From www.golighthouse.com
Cleanroom Classifications Explained Lighthouse Worldwide Solutions Clean Room Classification As Per Who This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. The who expert committee on specifi cations for. One that would consist of guidelines containing recommendations for. It was agreed that the. Clean Room Classification As Per Who.
From www.mecart-cleanrooms.com
Cleanroom Classifications (ISO 8, ISO 7, ISO 6, ISO 5) Clean Room Classification As Per Who This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. It was agreed that the guidelines be amended to comprise two documents: Who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi cations for. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements. Clean Room Classification As Per Who.
From www.rdworldonline.com
How classification impacts the design of a cleanroom Research Clean Room Classification As Per Who For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. It was agreed that the guidelines be amended to comprise two documents: This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but. Clean Room Classification As Per Who.
From gmpinsiders.com
GMP Cleanroom Classifications Understand Class A, B, C And D Clean Room Classification As Per Who Who good practices for pharmaceutical microbiology laboratories. It was agreed that the guidelines be amended to comprise two documents: For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. Requirements for cleanrooms are among the most critical. Clean Room Classification As Per Who.
From ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Clean Room Classification As Per Who For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. Who good practices for pharmaceutical microbiology laboratories. One that would consist of guidelines containing recommendations for. It was agreed that the guidelines be amended to comprise two documents: Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps),. Clean Room Classification As Per Who.
From www.lmairtech.com
Cleanroom Classification & Design Guidelines LM AIR TECHNOLOGY Clean Room Classification As Per Who One that would consist of guidelines containing recommendations for. Who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi cations for. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. It was agreed that the guidelines be amended to comprise two documents: Requirements for cleanrooms are among the. Clean Room Classification As Per Who.
From ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Clean Room Classification As Per Who Who good practices for pharmaceutical microbiology laboratories. One that would consist of guidelines containing recommendations for. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. The who expert. Clean Room Classification As Per Who.
From ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Clean Room Classification As Per Who The who expert committee on specifi cations for. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. One that would consist of guidelines containing recommendations for. Who good practices for pharmaceutical. Clean Room Classification As Per Who.
From www.iqsdirectory.com
Cleanroom What is it? ISO Standards and Classifications, Design, Types Clean Room Classification As Per Who Who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi cations for. It was agreed that the guidelines be amended to comprise two documents: One that would consist of guidelines containing recommendations for. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. Requirements for cleanrooms are among the. Clean Room Classification As Per Who.
From www.studypool.com
SOLUTION 1 introduction classification of cleanroom Studypool Clean Room Classification As Per Who Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. One that would consist of guidelines containing recommendations for. The who expert committee on specifi cations for. It was agreed that the guidelines be amended to comprise two documents: Who good practices for pharmaceutical microbiology laboratories. This. Clean Room Classification As Per Who.
From vem-medical.com
How to Maintain Cleanrooms Clean Room Classification As Per Who It was agreed that the guidelines be amended to comprise two documents: The who expert committee on specifi cations for. For initial classification, or classification following significant change, it is recommended that both ≥0.5 μm and ≥5 μm. Who good practices for pharmaceutical microbiology laboratories. This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in.. Clean Room Classification As Per Who.
From us.ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Clean Room Classification As Per Who The who expert committee on specifi cations for. One that would consist of guidelines containing recommendations for. This document was prepared by technical committee iso/tc 209, cleanrooms and associated controlled environments, in. Requirements for cleanrooms are among the most critical issues of good manufacturing practices (gmps), but differences between requirements in the us and eu. For initial classification, or classification. Clean Room Classification As Per Who.