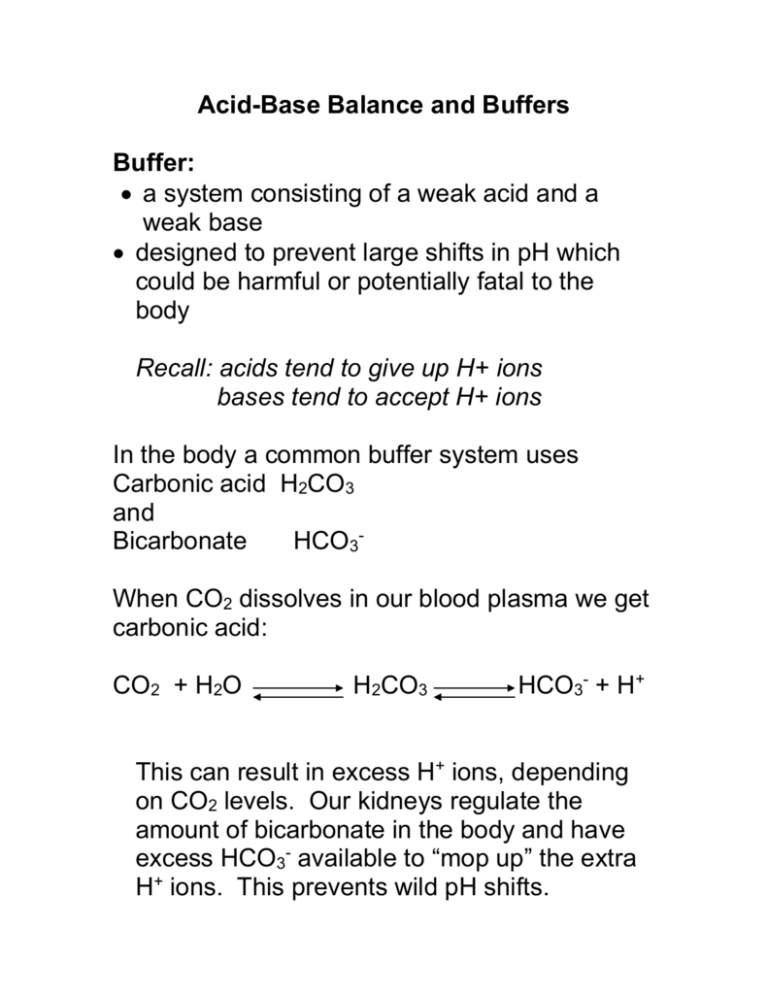
Buffer In Acid Base Balance . The ph buffer systems work. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. these weak acids and bases exist in pairs that are in balance under normal ph conditions. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.
from studylib.net
the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. these weak acids and bases exist in pairs that are in balance under normal ph conditions. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. The ph buffer systems work. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.
AcidBase Balance and Buffers
Buffer In Acid Base Balance these weak acids and bases exist in pairs that are in balance under normal ph conditions. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. these weak acids and bases exist in pairs that are in balance under normal ph conditions. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. The ph buffer systems work.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Buffer In Acid Base Balance intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph buffer systems work. Calculate the ph. Buffer In Acid Base Balance.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 Buffer In Acid Base Balance these weak acids and bases exist in pairs that are in balance under normal ph conditions. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood. Buffer In Acid Base Balance.
From philschatz.com
AcidBase Balance · Anatomy and Physiology Buffer In Acid Base Balance the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. these weak acids and bases exist in pairs that are in balance under normal ph conditions. The ph buffer systems work. the. Buffer In Acid Base Balance.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID1958325 Buffer In Acid Base Balance intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood. Buffer In Acid Base Balance.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 Buffer In Acid Base Balance the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. these weak acids and bases exist in pairs that are in balance under normal ph conditions. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. intracellular and extracellular buffers are the. Buffer In Acid Base Balance.
From fblt.cz
7. AcidBase Balance • Functions of Cells and Human Body Buffer In Acid Base Balance the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. these weak acids and bases exist in pairs that are in balance under normal ph conditions. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. The ph buffer systems work. Calculate the ph of. Buffer In Acid Base Balance.
From www.slideserve.com
PPT Fluid and Electrolyte Balance PowerPoint Presentation, free Buffer In Acid Base Balance intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. The ph buffer systems work. the. Buffer In Acid Base Balance.
From labpedia.net
Acidbase Balance Part 2 Introduction of AcidBase Balance Buffer In Acid Base Balance the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. these weak acids and bases exist in pairs that are in balance under normal ph conditions. the buffer systems functioning in blood. Buffer In Acid Base Balance.
From www.labpedia.net
Acidbase Balance Part 1 Metabolic acidosis, and Metabolic Buffer In Acid Base Balance The ph buffer systems work. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. these. Buffer In Acid Base Balance.
From dxoaxwzzm.blob.core.windows.net
Proteins Regulate The AcidBase Balance Of The Blood By at Richard Boyd Buffer In Acid Base Balance the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph buffer systems work. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Calculate. Buffer In Acid Base Balance.
From studylib.net
AcidBase Balance and Buffers Buffer In Acid Base Balance Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph buffer systems work. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. Buffer In Acid Base Balance.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free Buffer In Acid Base Balance these weak acids and bases exist in pairs that are in balance under normal ph conditions. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph buffer systems work. . Buffer In Acid Base Balance.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation ID496487 Buffer In Acid Base Balance Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. intracellular and extracellular buffers. Buffer In Acid Base Balance.
From labpedia.net
Acidbase Balance Part 1 A Introduction to the AcidBase Balance Buffer In Acid Base Balance these weak acids and bases exist in pairs that are in balance under normal ph conditions. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. intracellular and extracellular buffers are the. Buffer In Acid Base Balance.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID1714227 Buffer In Acid Base Balance Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. these weak acids and. Buffer In Acid Base Balance.
From labpedia.net
Acidbase Balance Part 1 A Introduction to the AcidBase Balance Buffer In Acid Base Balance The ph buffer systems work. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. these weak acids and bases exist in pairs that are in balance under normal ph conditions. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the. Buffer In Acid Base Balance.
From www.slideshare.net
Acid base balance Buffer In Acid Base Balance these weak acids and bases exist in pairs that are in balance under normal ph conditions. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. The ph buffer systems work. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems. Buffer In Acid Base Balance.
From www.youtube.com
1 Regulation of Blood pH By Buffer SystemsAcid Base Balance Buffer In Acid Base Balance the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. these weak acids and. Buffer In Acid Base Balance.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID2240409 Buffer In Acid Base Balance these weak acids and bases exist in pairs that are in balance under normal ph conditions. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. the buffer systems functioning in blood plasma. Buffer In Acid Base Balance.
From www.slideserve.com
PPT Acid and Base Balance PowerPoint Presentation, free download ID Buffer In Acid Base Balance the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. intracellular and extracellular buffers. Buffer In Acid Base Balance.
From kyvokedymolyb.modellervefiyatlar.com
Acid Base Buffers Buffer In Acid Base Balance Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. these weak acids and bases exist. Buffer In Acid Base Balance.
From www.studypool.com
SOLUTION Biochemistry acid base balance ph and buffers 1 Studypool Buffer In Acid Base Balance the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph buffer systems work. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. these weak. Buffer In Acid Base Balance.
From www.studypool.com
SOLUTION Ph buffers and acid base balance Studypool Buffer In Acid Base Balance intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. these weak acids and bases exist in pairs that are in balance under normal ph conditions. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include. Buffer In Acid Base Balance.
From www.slideserve.com
PPT Chapter 24 Water, Electrolyte and AcidBase Balance PowerPoint Buffer In Acid Base Balance Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph buffer systems work. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. Buffer In Acid Base Balance.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID2240409 Buffer In Acid Base Balance intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph buffer systems work. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Calculate the ph. Buffer In Acid Base Balance.
From www.online-sciences.com
Acidbase balance importance, Defense against changes in Hydrogen ion Buffer In Acid Base Balance the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. these weak acids and bases exist in pairs that are in balance under normal ph conditions. intracellular and extracellular buffers are the. Buffer In Acid Base Balance.
From www.slideserve.com
PPT Chapter 27 Fluid, Electrolyte and Acidbase Balance PowerPoint Buffer In Acid Base Balance intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. the buffer systems functioning in blood. Buffer In Acid Base Balance.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID1994730 Buffer In Acid Base Balance intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. The ph buffer systems work. these. Buffer In Acid Base Balance.
From doctorlib.info
Acidbase Balance Physiology An Illustrated Review Buffer In Acid Base Balance The ph buffer systems work. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. these weak acids and bases exist in pairs that are in balance under normal ph conditions. . Buffer In Acid Base Balance.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID1994730 Buffer In Acid Base Balance these weak acids and bases exist in pairs that are in balance under normal ph conditions. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood. Buffer In Acid Base Balance.
From www.anaesthesiajournal.co.uk
Renal physiology acidbase balance Anaesthesia & Intensive Care Medicine Buffer In Acid Base Balance the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. The ph buffer systems work. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. Buffer In Acid Base Balance.
From www.slideshare.net
Acid base balance Buffer In Acid Base Balance the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph buffer systems work. these weak acids and bases exist in pairs that are in balance under normal ph conditions. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the. Buffer In Acid Base Balance.
From www.youtube.com
Acid Base Physiology Part One Basics Buffers Renal Physiology Buffer In Acid Base Balance The ph buffer systems work. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Calculate the ph. Buffer In Acid Base Balance.
From www.paediatricsandchildhealthjournal.co.uk
Understanding blood gases/acidbase balance Paediatrics and Child Health Buffer In Acid Base Balance Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The ph buffer systems work. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. Buffer In Acid Base Balance.
From labpedia.net
Acidbase Balance Part 1 A Introduction to the AcidBase Balance Buffer In Acid Base Balance The ph buffer systems work. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. . Buffer In Acid Base Balance.