Magnesium Bicarbonate Solubility In Water . Mg (oh) 2 + 2co 2 → mg (hco 3) 2. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. It is highly soluble in water. solubility of inorganic compounds in water in relation to temperature. a solution of mg (hco 3) 2 commonly called magnesium bicarbonate water can be produced through the reaction of magnesium hydroxide (such as milk of magnesia) and pressurized carbon dioxide (like seltzer water) [4]: when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. Solubility table of 128 inorganic compounds in water at. according to this wikipedia solubility table, lithium bicarbonate is soluble in water to $\pu{5.7 g/100ml}$ at $\pu{20^oc}$, while this wikipedia page.
from www.chegg.com
when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. It is highly soluble in water. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of. according to this wikipedia solubility table, lithium bicarbonate is soluble in water to $\pu{5.7 g/100ml}$ at $\pu{20^oc}$, while this wikipedia page. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. a solution of mg (hco 3) 2 commonly called magnesium bicarbonate water can be produced through the reaction of magnesium hydroxide (such as milk of magnesia) and pressurized carbon dioxide (like seltzer water) [4]: Solubility table of 128 inorganic compounds in water at. solubility of inorganic compounds in water in relation to temperature.
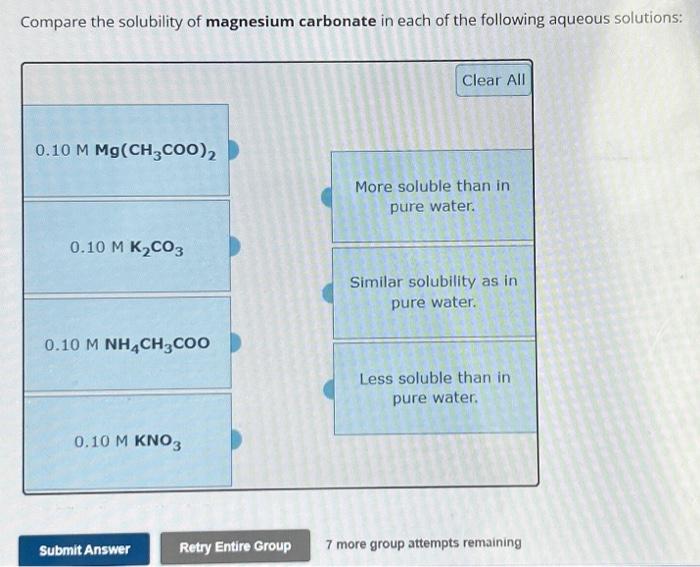
Solved Compare the solubility of magnesium carbonate in each
Magnesium Bicarbonate Solubility In Water according to this wikipedia solubility table, lithium bicarbonate is soluble in water to $\pu{5.7 g/100ml}$ at $\pu{20^oc}$, while this wikipedia page. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. It is highly soluble in water. solubility of inorganic compounds in water in relation to temperature. Solubility table of 128 inorganic compounds in water at. according to this wikipedia solubility table, lithium bicarbonate is soluble in water to $\pu{5.7 g/100ml}$ at $\pu{20^oc}$, while this wikipedia page. a solution of mg (hco 3) 2 commonly called magnesium bicarbonate water can be produced through the reaction of magnesium hydroxide (such as milk of magnesia) and pressurized carbon dioxide (like seltzer water) [4]: 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of. Mg (oh) 2 + 2co 2 → mg (hco 3) 2.
From chemistry.stackexchange.com
chemistry Are lithium bicarbonate and magnesium bicarbonate Magnesium Bicarbonate Solubility In Water according to this wikipedia solubility table, lithium bicarbonate is soluble in water to $\pu{5.7 g/100ml}$ at $\pu{20^oc}$, while this wikipedia page. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. It is highly soluble. Magnesium Bicarbonate Solubility In Water.
From www.youtube.com
Magnesium Bicarbonate Water (How I Make It) YouTube Magnesium Bicarbonate Solubility In Water a solution of mg (hco 3) 2 commonly called magnesium bicarbonate water can be produced through the reaction of magnesium hydroxide (such as milk of magnesia) and pressurized carbon dioxide (like seltzer water) [4]: solubility of inorganic compounds in water in relation to temperature. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate. Magnesium Bicarbonate Solubility In Water.
From pubs.rsc.org
Solubility investigations in the amorphous calcium magnesium carbonate Magnesium Bicarbonate Solubility In Water 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. solubility of inorganic compounds in water in relation to temperature. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout. Magnesium Bicarbonate Solubility In Water.
From www.chegg.com
Solved Compare the solubility of magnesium carbonate in each Magnesium Bicarbonate Solubility In Water solubility of inorganic compounds in water in relation to temperature. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. a solution of. Magnesium Bicarbonate Solubility In Water.
From calebcroomphysci4dummies.weebly.com
Solubility Physical Science For Dummies Magnesium Bicarbonate Solubility In Water It is highly soluble in water. according to this wikipedia solubility table, lithium bicarbonate is soluble in water to $\pu{5.7 g/100ml}$ at $\pu{20^oc}$, while this wikipedia page. solubility of inorganic compounds in water in relation to temperature. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. a solution of. Magnesium Bicarbonate Solubility In Water.
From www.youtube.com
The molar solubility of magnesium carbonate is 1.87x10^4 mol/L Magnesium Bicarbonate Solubility In Water solubility of inorganic compounds in water in relation to temperature. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. Solubility table of 128 inorganic compounds in water at. a solution of mg (hco 3) 2 commonly called magnesium bicarbonate water can be produced through the reaction of magnesium hydroxide (such as milk of magnesia) and pressurized. Magnesium Bicarbonate Solubility In Water.
From www.chegg.com
Solved Compare the solubility of magnesium carbonate in each Magnesium Bicarbonate Solubility In Water a solution of mg (hco 3) 2 commonly called magnesium bicarbonate water can be produced through the reaction of magnesium hydroxide (such as milk of magnesia) and pressurized carbon dioxide (like seltzer water) [4]: Mg (oh) 2 + 2co 2 → mg (hco 3) 2. It is highly soluble in water. solubility of inorganic compounds in water in. Magnesium Bicarbonate Solubility In Water.
From www.researchgate.net
2) gives the solubility of magnesium in water at 25°C as a function of Magnesium Bicarbonate Solubility In Water Mg (oh) 2 + 2co 2 → mg (hco 3) 2. according to this wikipedia solubility table, lithium bicarbonate is soluble in water to $\pu{5.7 g/100ml}$ at $\pu{20^oc}$, while this wikipedia page. It is highly soluble in water. Solubility table of 128 inorganic compounds in water at. solubility of inorganic compounds in water in relation to temperature. . Magnesium Bicarbonate Solubility In Water.
From www.researchgate.net
MgCO3 mineral solubility at various temperatures, pressures, NaCl Magnesium Bicarbonate Solubility In Water according to this wikipedia solubility table, lithium bicarbonate is soluble in water to $\pu{5.7 g/100ml}$ at $\pu{20^oc}$, while this wikipedia page. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. It is highly soluble. Magnesium Bicarbonate Solubility In Water.
From byjus.com
Solubility product of magnesium Hydroxide is 4 10 12 . The number of Magnesium Bicarbonate Solubility In Water according to this wikipedia solubility table, lithium bicarbonate is soluble in water to $\pu{5.7 g/100ml}$ at $\pu{20^oc}$, while this wikipedia page. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of.. Magnesium Bicarbonate Solubility In Water.
From www.degruyter.com
Synthesis of magnesium carbonate hydrate from natural talc Magnesium Bicarbonate Solubility In Water Solubility table of 128 inorganic compounds in water at. It is highly soluble in water. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. solubility of inorganic compounds in water in relation to temperature. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. a solution of mg (hco. Magnesium Bicarbonate Solubility In Water.
From www.chemistrylearner.com
Magnesium Bicarbonate Facts, Formula, Synthesis, Properties, Uses Magnesium Bicarbonate Solubility In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. according to this wikipedia solubility table, lithium bicarbonate is soluble in water to $\pu{5.7 g/100ml}$ at $\pu{20^oc}$, while this wikipedia page. a solution of. Magnesium Bicarbonate Solubility In Water.
From oneclass.com
OneClass of the magnesium carbonate in Compare the solubility Magnesium Bicarbonate Solubility In Water according to this wikipedia solubility table, lithium bicarbonate is soluble in water to $\pu{5.7 g/100ml}$ at $\pu{20^oc}$, while this wikipedia page. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. It is highly soluble in water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Magnesium Bicarbonate Solubility In Water.
From www.slideserve.com
PPT Magnesium Bicarbonate Water PowerPoint Presentation, free Magnesium Bicarbonate Solubility In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. a solution of mg (hco 3) 2 commonly called magnesium bicarbonate water can be. Magnesium Bicarbonate Solubility In Water.
From learningcampusdirk.z13.web.core.windows.net
Solubility Chart In Water Magnesium Bicarbonate Solubility In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of. Mg (oh) 2 + 2co. Magnesium Bicarbonate Solubility In Water.
From www.nourishthrive.com
Magnesium Bicarbonate Water Recipe NourishThrive Magnesium Bicarbonate Solubility In Water solubility of inorganic compounds in water in relation to temperature. Solubility table of 128 inorganic compounds in water at. It is highly soluble in water. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of. magnesium. Magnesium Bicarbonate Solubility In Water.
From mungfali.com
Solubility Rules Flowchart Chart Chemistry Magnesium Bicarbonate Solubility In Water 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. a solution of mg (hco 3) 2 commonly called magnesium bicarbonate water can be produced through the reaction of magnesium hydroxide (such as milk of magnesia) and. Magnesium Bicarbonate Solubility In Water.
From www.chegg.com
Solved Determine the solubility of magnesium hydroxide? See Magnesium Bicarbonate Solubility In Water magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. Solubility table of 128 inorganic compounds in water at. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. solubility of inorganic compounds in water in relation to temperature. 133 rows in this section we will apply chemical equilibria to. Magnesium Bicarbonate Solubility In Water.
From sciencenotes.org
Solubility Rules Chart and Memorization Tips Magnesium Bicarbonate Solubility In Water It is highly soluble in water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. according to this wikipedia solubility table, lithium bicarbonate. Magnesium Bicarbonate Solubility In Water.
From www.chegg.com
Solved Compare the solubility of magnesium carbonate in each Magnesium Bicarbonate Solubility In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. a solution of mg (hco 3) 2 commonly called magnesium bicarbonate water can be produced through the reaction of magnesium hydroxide (such as milk of. Magnesium Bicarbonate Solubility In Water.
From oneclass.com
OneClass of the magnesium carbonate in Compare the solubility Magnesium Bicarbonate Solubility In Water It is highly soluble in water. solubility of inorganic compounds in water in relation to temperature. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of. Solubility table of 128 inorganic compounds in water at. a. Magnesium Bicarbonate Solubility In Water.
From www.slideserve.com
PPT Dissolution and Precipitation PowerPoint Presentation, free Magnesium Bicarbonate Solubility In Water a solution of mg (hco 3) 2 commonly called magnesium bicarbonate water can be produced through the reaction of magnesium hydroxide (such as milk of magnesia) and pressurized carbon dioxide (like seltzer water) [4]: Mg (oh) 2 + 2co 2 → mg (hco 3) 2. 133 rows in this section we will apply chemical equilibria to the concept. Magnesium Bicarbonate Solubility In Water.
From www.numerade.com
SOLVED The molar solubility of magnesium carbonate in a water solution Magnesium Bicarbonate Solubility In Water Mg (oh) 2 + 2co 2 → mg (hco 3) 2. a solution of mg (hco 3) 2 commonly called magnesium bicarbonate water can be produced through the reaction of magnesium hydroxide (such as milk of magnesia) and pressurized carbon dioxide (like seltzer water) [4]: solubility of inorganic compounds in water in relation to temperature. It is highly. Magnesium Bicarbonate Solubility In Water.
From pubs.acs.org
Control of Water Chemistry in Alkaline Lakes Solubility of Magnesium Bicarbonate Solubility In Water Mg (oh) 2 + 2co 2 → mg (hco 3) 2. a solution of mg (hco 3) 2 commonly called magnesium bicarbonate water can be produced through the reaction of magnesium hydroxide (such as milk of magnesia) and pressurized carbon dioxide (like seltzer water) [4]: according to this wikipedia solubility table, lithium bicarbonate is soluble in water to. Magnesium Bicarbonate Solubility In Water.
From oneclass.com
OneClass of the magnesium carbonate in Compare the solubility Magnesium Bicarbonate Solubility In Water solubility of inorganic compounds in water in relation to temperature. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. 133 rows in this section we will apply chemical equilibria to the concept of. Magnesium Bicarbonate Solubility In Water.
From www.chegg.com
Solved 18. What is the solubility of magnesium carbonate, Magnesium Bicarbonate Solubility In Water 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of. It is highly soluble in water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between. Magnesium Bicarbonate Solubility In Water.
From www.flinnsci.com
Solubility Rules Chart Flinn Scientific Magnesium Bicarbonate Solubility In Water magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. solubility of inorganic compounds in water in relation to temperature. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of.. Magnesium Bicarbonate Solubility In Water.
From www.chegg.com
Solved Compare the solubility of magnesium carbonate in each Magnesium Bicarbonate Solubility In Water Solubility table of 128 inorganic compounds in water at. a solution of mg (hco 3) 2 commonly called magnesium bicarbonate water can be produced through the reaction of magnesium hydroxide (such as milk of magnesia) and pressurized carbon dioxide (like seltzer water) [4]: when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Magnesium Bicarbonate Solubility In Water.
From www.vedantu.com
Magnesium Bicarbonate Learn Important Terms and Concepts Magnesium Bicarbonate Solubility In Water Solubility table of 128 inorganic compounds in water at. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of. a. Magnesium Bicarbonate Solubility In Water.
From www.plantprod.com
Adjusting Irrigation Water Bicarbonates vs. pH Master PlantProd Inc. Magnesium Bicarbonate Solubility In Water magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. It is highly soluble in water. solubility of inorganic compounds in water in relation to temperature. 133 rows in this section we will apply chemical equilibria to the concept of. Magnesium Bicarbonate Solubility In Water.
From www.numerade.com
SOLVED Magnesium carbonate, MgCO3, is a salt of low solubility. When Magnesium Bicarbonate Solubility In Water solubility of inorganic compounds in water in relation to temperature. according to this wikipedia solubility table, lithium bicarbonate is soluble in water to $\pu{5.7 g/100ml}$ at $\pu{20^oc}$, while this wikipedia page. It is highly soluble in water. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of.. Magnesium Bicarbonate Solubility In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Magnesium Bicarbonate Solubility In Water It is highly soluble in water. solubility of inorganic compounds in water in relation to temperature. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2,. Magnesium Bicarbonate Solubility In Water.
From www.chegg.com
Solved Calculate the solubility of magnesium carbonate at 25 Magnesium Bicarbonate Solubility In Water 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of. according to this wikipedia solubility table, lithium bicarbonate is soluble in water to $\pu{5.7 g/100ml}$ at $\pu{20^oc}$, while this wikipedia page. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium.. Magnesium Bicarbonate Solubility In Water.
From www.chegg.com
Solved Compare the solubility of magnesium carbonate in each Magnesium Bicarbonate Solubility In Water Mg (oh) 2 + 2co 2 → mg (hco 3) 2. magnesium bicarbonate or magnesium hydrogencarbonate, mg (h co 3) 2, is the bicarbonate salt of magnesium. It is highly soluble in water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the. Magnesium Bicarbonate Solubility In Water.
From www.numerade.com
SOLVEDMagnesium carbonate is slighlly soluble salt with Kie 6.82 x 108 Magnesium Bicarbonate Solubility In Water Solubility table of 128 inorganic compounds in water at. Mg (oh) 2 + 2co 2 → mg (hco 3) 2. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of. a solution of mg (hco 3) 2 commonly called magnesium bicarbonate water can be produced through the reaction. Magnesium Bicarbonate Solubility In Water.