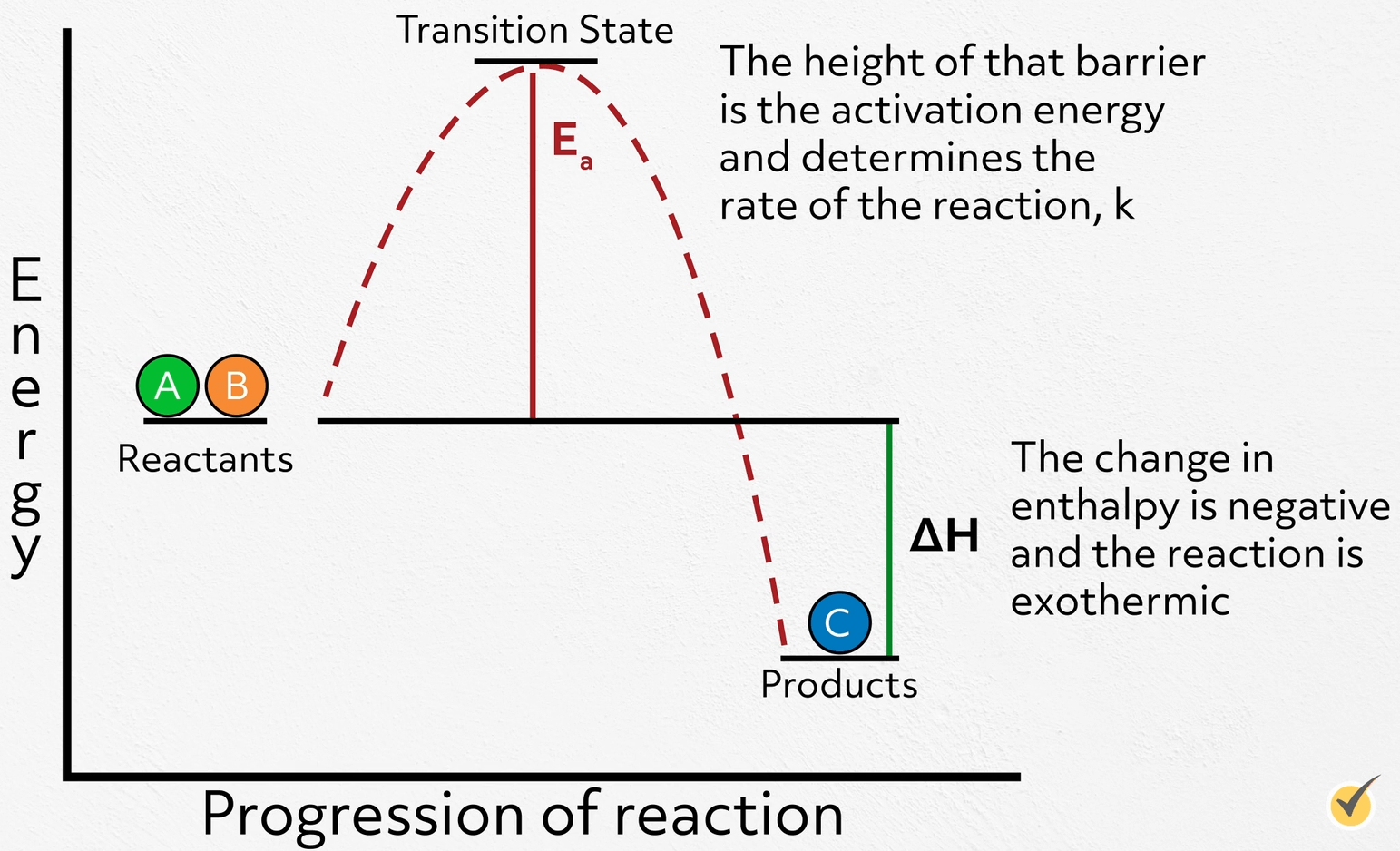
Catalysts Decrease Reaction Rates . This page explains how adding a catalyst affects the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst affects the reaction rate without being used up. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding within reactants, or donate electron density to reactants. A catalyst reduces the activation energy needed for a reaction. The presence of a catalyst doesn’t change a chemical reaction, but it does help it reach equilibrium more quickly. However, not all reactions have suitable catalysts. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. Only a very small amount of catalyst. In this section, we will examine the three major classes of catalysts:
from www.mometrix.com
Heterogeneous catalysts, homogeneous catalysts, and enzymes. However, not all reactions have suitable catalysts. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. A catalyst affects the reaction rate without being used up. Only a very small amount of catalyst. This page describes and explains the way that adding a catalyst affects the rate of a reaction. The presence of a catalyst doesn’t change a chemical reaction, but it does help it reach equilibrium more quickly. In this section, we will examine the three major classes of catalysts: A catalyst reduces the activation energy needed for a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction.
What is a Catalyst? Chemistry Review (Video)
Catalysts Decrease Reaction Rates Heterogeneous catalysts, homogeneous catalysts, and enzymes. The presence of a catalyst doesn’t change a chemical reaction, but it does help it reach equilibrium more quickly. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst affects the reaction rate without being used up. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. It assumes that you are already familiar with basic ideas about the collision theory of reaction. In this section, we will examine the three major classes of catalysts: Only a very small amount of catalyst. This page explains how adding a catalyst affects the rate of a reaction. Catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding within reactants, or donate electron density to reactants. A catalyst reduces the activation energy needed for a reaction. However, not all reactions have suitable catalysts. A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction.
From byjus.com
How does a catalyst increase the rate of a reaction? Catalysts Decrease Reaction Rates A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. A catalyst affects the reaction rate without being used up. Heterogeneous catalysts, homogeneous catalysts, and enzymes. However, not all reactions have suitable catalysts. This page explains how adding a catalyst affects the rate of a reaction. A catalyst reduces the. Catalysts Decrease Reaction Rates.
From www.chemicals.co.uk
Chemistry GCSE Revision The Rate and Extent of Chemical Change Catalysts Decrease Reaction Rates However, not all reactions have suitable catalysts. Catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding within reactants, or donate electron density to reactants. A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. It assumes that you are already familiar with basic ideas about. Catalysts Decrease Reaction Rates.
From www.slideserve.com
PPT Reaction Rates and Equilibrium PowerPoint Presentation, free Catalysts Decrease Reaction Rates Only a very small mass of catalyst is needed to increase the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. In this section, we will examine the three major classes of catalysts: This page explains how adding a catalyst affects the rate of a reaction. The presence. Catalysts Decrease Reaction Rates.
From www.slideshare.net
Rate of reaction(f5) Catalysts Decrease Reaction Rates Only a very small amount of catalyst. Heterogeneous catalysts, homogeneous catalysts, and enzymes. This page describes and explains the way that adding a catalyst affects the rate of a reaction. However, not all reactions have suitable catalysts. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. Catalysts Decrease Reaction Rates.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Catalysts Decrease Reaction Rates Only a very small mass of catalyst is needed to increase the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. A catalyst reduces the activation energy needed for a reaction. The presence of a catalyst doesn’t change a chemical reaction, but it does help it reach equilibrium more quickly. Catalysts increase. Catalysts Decrease Reaction Rates.
From studyfullfarrior.z19.web.core.windows.net
Rates Of Reaction And Energy Changes Catalysts Decrease Reaction Rates In this section, we will examine the three major classes of catalysts: The presence of a catalyst doesn’t change a chemical reaction, but it does help it reach equilibrium more quickly. This page explains how adding a catalyst affects the rate of a reaction. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates. Catalysts Decrease Reaction Rates.
From www.slideserve.com
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687 Catalysts Decrease Reaction Rates Only a very small amount of catalyst. A catalyst reduces the activation energy needed for a reaction. In this section, we will examine the three major classes of catalysts: Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. The presence of. Catalysts Decrease Reaction Rates.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint Catalysts Decrease Reaction Rates Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. Catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce. Catalysts Decrease Reaction Rates.
From slideplayer.com
A catalyst lowers activation energy. ppt download Catalysts Decrease Reaction Rates In this section, we will examine the three major classes of catalysts: Heterogeneous catalysts, homogeneous catalysts, and enzymes. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. However, not all reactions have suitable catalysts. A catalyst is a substance. Catalysts Decrease Reaction Rates.
From www.slideserve.com
PPT Factors Affecting Reaction Rates PowerPoint Presentation, free Catalysts Decrease Reaction Rates It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page explains how adding a catalyst affects the rate of a reaction. A catalyst reduces the activation energy needed for a reaction. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. Catalysts Decrease Reaction Rates.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalysts Decrease Reaction Rates This page explains how adding a catalyst affects the rate of a reaction. Only a very small amount of catalyst. Only a very small mass of catalyst is needed to increase the rate of a reaction. The presence of a catalyst doesn’t change a chemical reaction, but it does help it reach equilibrium more quickly. A catalyst reduces the activation. Catalysts Decrease Reaction Rates.
From study.com
Effect of Catalysts on Rates of Reaction Video & Lesson Transcript Catalysts Decrease Reaction Rates It assumes that you are already familiar with basic ideas about the collision theory of reaction. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. In this section, we will examine the three major classes of catalysts: Heterogeneous catalysts, homogeneous catalysts,. Catalysts Decrease Reaction Rates.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts Decrease Reaction Rates The presence of a catalyst doesn’t change a chemical reaction, but it does help it reach equilibrium more quickly. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. Heterogeneous catalysts, homogeneous catalysts, and enzymes. It assumes that you are already familiar. Catalysts Decrease Reaction Rates.
From slideplayer.com
Chemical Reactions and Collision Theory ppt download Catalysts Decrease Reaction Rates Heterogeneous catalysts, homogeneous catalysts, and enzymes. Only a very small amount of catalyst. The presence of a catalyst doesn’t change a chemical reaction, but it does help it reach equilibrium more quickly. In this section, we will examine the three major classes of catalysts: This page describes and explains the way that adding a catalyst affects the rate of a. Catalysts Decrease Reaction Rates.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalysts Decrease Reaction Rates Only a very small mass of catalyst is needed to increase the rate of a reaction. In this section, we will examine the three major classes of catalysts: This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding within. Catalysts Decrease Reaction Rates.
From www.slideserve.com
PPT Reaction Rates & Equilibrium PowerPoint Presentation, free Catalysts Decrease Reaction Rates Only a very small mass of catalyst is needed to increase the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. Only a very small amount of catalyst. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Catalysts increase the frequency of collisions between reactants,. Catalysts Decrease Reaction Rates.
From www.nagwa.com
Question Video Understanding How Catalysts Affect the Rates and the Catalysts Decrease Reaction Rates It assumes that you are already familiar with basic ideas about the collision theory of reaction. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. A catalyst is a substance which changes the rate of reaction but is unchanged at the. Catalysts Decrease Reaction Rates.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalysts Decrease Reaction Rates It assumes that you are already familiar with basic ideas about the collision theory of reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. In this section, we will examine the three major classes of catalysts: A catalyst affects the reaction rate without being used up. A catalyst reduces the activation energy. Catalysts Decrease Reaction Rates.
From slideplayer.com
Factors Affecting Reaction Rates ppt download Catalysts Decrease Reaction Rates This page explains how adding a catalyst affects the rate of a reaction. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes. Catalysts Decrease Reaction Rates.
From 2012books.lardbucket.org
Catalysis Catalysts Decrease Reaction Rates However, not all reactions have suitable catalysts. A catalyst reduces the activation energy needed for a reaction. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision. Catalysts Decrease Reaction Rates.
From helecu.com
Factors Affecting Reaction Rates Chemistry Atoms First (2022) Catalysts Decrease Reaction Rates Only a very small amount of catalyst. A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that. Catalysts Decrease Reaction Rates.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Catalysts Decrease Reaction Rates Only a very small amount of catalyst. Catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding within reactants, or donate electron density to reactants. The presence of a catalyst doesn’t change a chemical reaction, but it does help it reach equilibrium more quickly. Only a very small mass of catalyst is needed to increase the. Catalysts Decrease Reaction Rates.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Catalysts Decrease Reaction Rates Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. Only a very small amount of catalyst. Heterogeneous catalysts, homogeneous catalysts, and enzymes. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Only a very. Catalysts Decrease Reaction Rates.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID1785599 Catalysts Decrease Reaction Rates This page explains how adding a catalyst affects the rate of a reaction. Catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding within reactants, or donate electron density to reactants. In this section, we will examine the three major classes of catalysts: The presence of a catalyst doesn’t change a chemical reaction, but it does. Catalysts Decrease Reaction Rates.
From slideplayer.com
Reaction Rates and Chemical Equilibrium ppt download Catalysts Decrease Reaction Rates This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. Catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding within reactants, or donate electron density to reactants. The. Catalysts Decrease Reaction Rates.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalysts Decrease Reaction Rates Only a very small amount of catalyst. A catalyst reduces the activation energy needed for a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. A catalyst affects the reaction rate without being used up. Catalysts. Catalysts Decrease Reaction Rates.
From slideplayer.com
and Reactors Review ppt download Catalysts Decrease Reaction Rates The presence of a catalyst doesn’t change a chemical reaction, but it does help it reach equilibrium more quickly. Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst reduces the activation energy needed for a reaction. Catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding within reactants, or donate electron density to reactants. This page describes. Catalysts Decrease Reaction Rates.
From www.researchgate.net
Plots of ln(C0/Ct) vs. the reaction time and apparent rate constants Catalysts Decrease Reaction Rates Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. However, not all reactions have suitable catalysts. Only a very small amount of catalyst. A catalyst affects the reaction rate without being used up. The presence of a catalyst doesn’t change a. Catalysts Decrease Reaction Rates.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 Catalysts Decrease Reaction Rates However, not all reactions have suitable catalysts. The presence of a catalyst doesn’t change a chemical reaction, but it does help it reach equilibrium more quickly. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Only a very small mass of catalyst is needed to increase the rate of a reaction. Only a very small amount of catalyst. It assumes that you are. Catalysts Decrease Reaction Rates.
From ppt-online.org
Rates of reaction презентация онлайн Catalysts Decrease Reaction Rates This page describes and explains the way that adding a catalyst affects the rate of a reaction. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. A catalyst is a substance which changes the rate of reaction but is unchanged at. Catalysts Decrease Reaction Rates.
From www.onlinebiologynotes.com
Rate of enzyme reactions and factor affecting the rate of enzyme Catalysts Decrease Reaction Rates A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse. Catalysts Decrease Reaction Rates.
From slideplayer.com
Factors Affecting Reaction Rates ppt download Catalysts Decrease Reaction Rates Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst affects the reaction rate without being used up. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Only a very small amount of catalyst. However, not all reactions have suitable catalysts. Catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding. Catalysts Decrease Reaction Rates.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation Catalysts Decrease Reaction Rates Only a very small amount of catalyst. The presence of a catalyst doesn’t change a chemical reaction, but it does help it reach equilibrium more quickly. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst affects the reaction rate without being used up. However, not all reactions have suitable catalysts. Catalysts. Catalysts Decrease Reaction Rates.
From www.slideserve.com
PPT Rates of Reaction PowerPoint Presentation, free download ID3014435 Catalysts Decrease Reaction Rates Catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding within reactants, or donate electron density to reactants. Only a very small mass of catalyst is needed to increase the rate of a reaction. In this section, we will examine the three major classes of catalysts: Only a very small amount of catalyst. This page describes. Catalysts Decrease Reaction Rates.
From www.slideserve.com
PPT Chemical Reactions and Collision Theory PowerPoint Presentation Catalysts Decrease Reaction Rates It assumes that you are already familiar with basic ideas about the collision theory of reaction. In this section, we will examine the three major classes of catalysts: Only a very small amount of catalyst. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst reduces. Catalysts Decrease Reaction Rates.