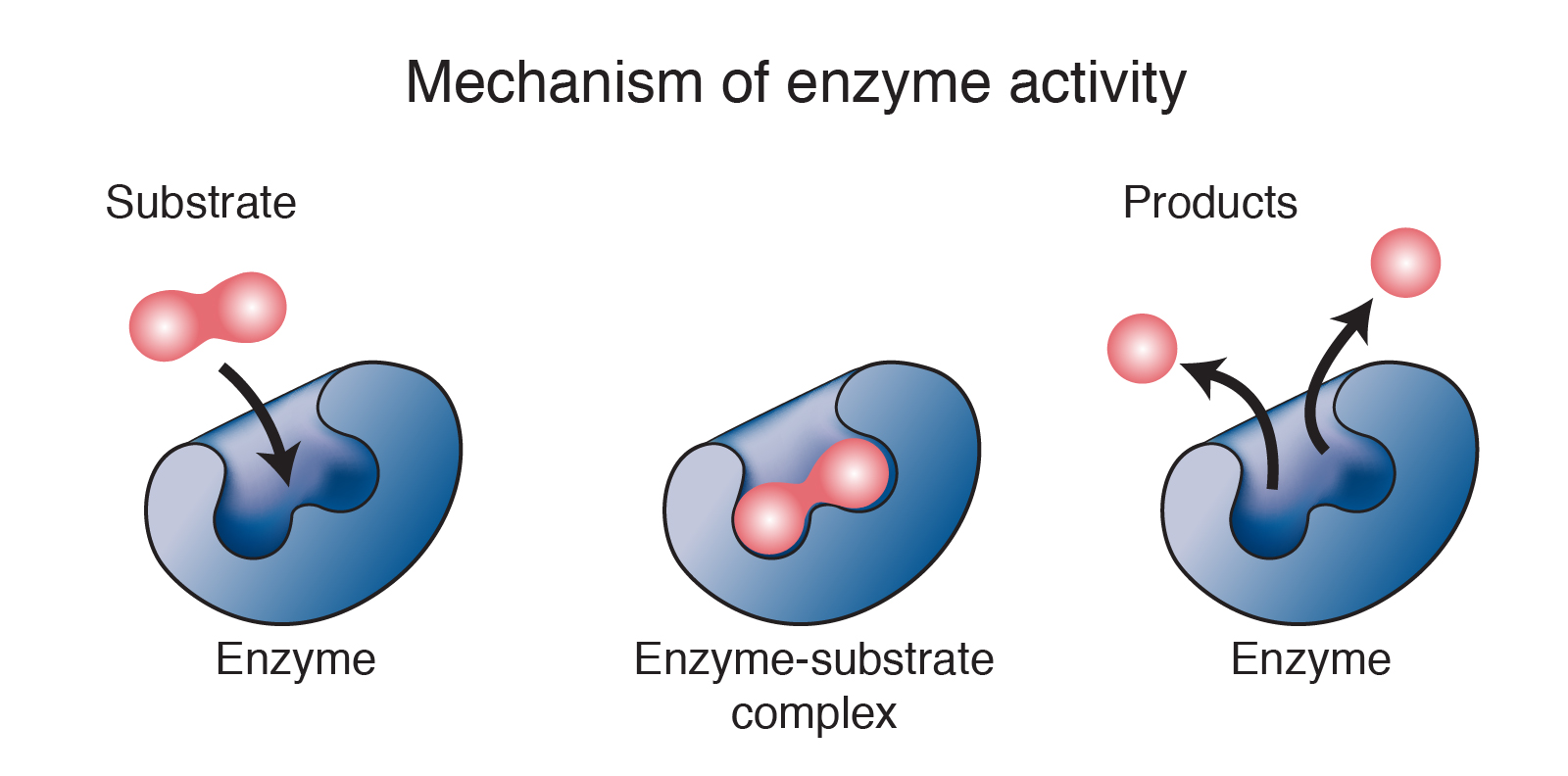
Enzymes Are Zero Order In A Rate Expression . For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. We refer to these reactions as. V is zero order with respect to s and first order in e. Why would you need a rate law? Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. Its differential rate law is rate = k.
from www.genome.gov
V is zero order with respect to s and first order in e. Its differential rate law is rate = k. We refer to these reactions as. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Why would you need a rate law? For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly.
Enzyme
Enzymes Are Zero Order In A Rate Expression Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. We refer to these reactions as. V is zero order with respect to s and first order in e. Its differential rate law is rate = k. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. Why would you need a rate law? Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly.
From www.slideserve.com
PPT 144 ZeroOrder Reactions PowerPoint Presentation, free download Enzymes Are Zero Order In A Rate Expression Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Its differential rate law is rate = k. We refer to these reactions as. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. Why would you need a rate law? Enzymes. Enzymes Are Zero Order In A Rate Expression.
From ibiologia.com
Enzymes Definition, Classification & Functions Enzymes Are Zero Order In A Rate Expression Its differential rate law is rate = k. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. Why would you need a rate law? V is zero order with respect to s and first order in e. For enzymatic reactions (or any catalytic reactions. Enzymes Are Zero Order In A Rate Expression.
From www.tessshebaylo.com
Rate Constant Equation For Zero Order Tessshebaylo Enzymes Are Zero Order In A Rate Expression Its differential rate law is rate = k. We refer to these reactions as. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it. Enzymes Are Zero Order In A Rate Expression.
From www.toppr.com
Derive an integrated rate equation for rate constant of a zero order Enzymes Are Zero Order In A Rate Expression Why would you need a rate law? For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. We refer to these reactions as. V is zero order with respect to s and first order in e. Enzyme kinetics is a key aspect of biochemistry that. Enzymes Are Zero Order In A Rate Expression.
From www.researchgate.net
Progress of a typical enzymatic reaction (a) The states of an enzyme Enzymes Are Zero Order In A Rate Expression Why would you need a rate law? Its differential rate law is rate = k. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in. Enzymes Are Zero Order In A Rate Expression.
From scienceinfo.com
Zeroorder Reaction Rate Equation, Unit, Graph, Example Enzymes Are Zero Order In A Rate Expression Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. We refer to these reactions as. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. Why would you need. Enzymes Are Zero Order In A Rate Expression.
From www.youtube.com
CHEMICAL integrated rate equation for zero order reaction Enzymes Are Zero Order In A Rate Expression Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. We refer to these reactions as. V is zero order with respect to s and first order in e. Its differential rate law is rate = k. Enzyme kinetics is a key aspect of biochemistry. Enzymes Are Zero Order In A Rate Expression.
From www.youtube.com
graph of integrated rate equation of zero order reaction (chemical Enzymes Are Zero Order In A Rate Expression Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. We refer to these reactions as. V is zero order with respect to s and first order in e. Its differential rate law is rate = k. For enzymatic reactions (or any catalytic reactions in. Enzymes Are Zero Order In A Rate Expression.
From www.onlinebiologynotes.com
Rate of enzyme reactions and factor affecting the rate of enzyme Enzymes Are Zero Order In A Rate Expression Its differential rate law is rate = k. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. V is zero order with respect to s and first order in e. Why would you need a rate law? We refer to these reactions as. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of. Enzymes Are Zero Order In A Rate Expression.
From www.youtube.com
Enzyme Specificity & Types of Enzyme Specificity YouTube Enzymes Are Zero Order In A Rate Expression Its differential rate law is rate = k. We refer to these reactions as. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. Why would you need a rate law? Enzyme kinetics is a key aspect of biochemistry that examines the rates of. For. Enzymes Are Zero Order In A Rate Expression.
From www.worthington-biochem.com
Enzyme Concentration Worthington Biochemical Enzymes Are Zero Order In A Rate Expression For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. V is zero order with respect to s and first. Enzymes Are Zero Order In A Rate Expression.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID999616 Enzymes Are Zero Order In A Rate Expression We refer to these reactions as. V is zero order with respect to s and first order in e. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Why would you. Enzymes Are Zero Order In A Rate Expression.
From chempedia.info
Enzyme activity curve Big Chemical Encyclopedia Enzymes Are Zero Order In A Rate Expression V is zero order with respect to s and first order in e. Its differential rate law is rate = k. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the. Enzymes Are Zero Order In A Rate Expression.
From ncertmcq.com
Enzymes Definition and its Types NCERT MCQ Enzymes Are Zero Order In A Rate Expression Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. Why would you need a rate law? Its differential rate. Enzymes Are Zero Order In A Rate Expression.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID196477 Enzymes Are Zero Order In A Rate Expression Why would you need a rate law? We refer to these reactions as. V is zero order with respect to s and first order in e. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. Its differential rate law is rate = k. For. Enzymes Are Zero Order In A Rate Expression.
From www.researchgate.net
Zeroorder ultrasensitivity at low enzyme concentrations for system (3 Enzymes Are Zero Order In A Rate Expression V is zero order with respect to s and first order in e. Why would you need a rate law? We refer to these reactions as. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it. Enzymes Are Zero Order In A Rate Expression.
From chem.libretexts.org
14.4 ZeroOrder Reactions Chemistry LibreTexts Enzymes Are Zero Order In A Rate Expression We refer to these reactions as. Why would you need a rate law? For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. Its differential rate law is rate = k. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. V. Enzymes Are Zero Order In A Rate Expression.
From lessoncampusunspelt.z13.web.core.windows.net
Enzyme Graph Examples Enzymes Are Zero Order In A Rate Expression We refer to these reactions as. Why would you need a rate law? Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. For enzymatic reactions (or any catalytic reactions in general),. Enzymes Are Zero Order In A Rate Expression.
From chemistryguru.com.sg
Rate Concentration Graph for Enzyme Catalysed Reaction Enzymes Are Zero Order In A Rate Expression V is zero order with respect to s and first order in e. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. We refer to these reactions as. Its differential rate. Enzymes Are Zero Order In A Rate Expression.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID196477 Enzymes Are Zero Order In A Rate Expression V is zero order with respect to s and first order in e. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is. Enzymes Are Zero Order In A Rate Expression.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID196477 Enzymes Are Zero Order In A Rate Expression V is zero order with respect to s and first order in e. Its differential rate law is rate = k. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. We refer to these reactions as. Why would you need a rate law? Enzyme. Enzymes Are Zero Order In A Rate Expression.
From www.genome.gov
Enzyme Enzymes Are Zero Order In A Rate Expression Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. Its differential rate law is rate = k. We refer to these reactions as. V is zero order with respect to s and first order in e. For enzymatic reactions (or any catalytic reactions in. Enzymes Are Zero Order In A Rate Expression.
From www.pinterest.com
Biochemistry 9.2 Enzyme part 1 Enzyme Enzymes Are Zero Order In A Rate Expression V is zero order with respect to s and first order in e. Why would you need a rate law? Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Its differential rate law is rate = k. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s],. Enzymes Are Zero Order In A Rate Expression.
From www.researchgate.net
5 Enzymes increase the rate of reaction Download Scientific Diagram Enzymes Are Zero Order In A Rate Expression Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Why would you need a rate law? Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. We refer to these reactions as. V is zero order with respect to s and. Enzymes Are Zero Order In A Rate Expression.
From www.slideserve.com
PPT Chemical Reactions & Enzymes PowerPoint Presentation, free Enzymes Are Zero Order In A Rate Expression Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. V is zero order with respect to s and first order in e. Why would you need a rate law? Enzyme kinetics is a key aspect of biochemistry that examines the rates of. We refer. Enzymes Are Zero Order In A Rate Expression.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID6630235 Enzymes Are Zero Order In A Rate Expression For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. We refer to these reactions as. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and. Enzymes Are Zero Order In A Rate Expression.
From www.tessshebaylo.com
Rate Constant Equation For Zero Order Tessshebaylo Enzymes Are Zero Order In A Rate Expression Enzyme kinetics is a key aspect of biochemistry that examines the rates of. V is zero order with respect to s and first order in e. Why would you need a rate law? We refer to these reactions as. Its differential rate law is rate = k. For enzymatic reactions (or any catalytic reactions in general), the initial rate of. Enzymes Are Zero Order In A Rate Expression.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Enzymes Are Zero Order In A Rate Expression V is zero order with respect to s and first order in e. Its differential rate law is rate = k. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. Why. Enzymes Are Zero Order In A Rate Expression.
From jackwestin.com
Dependence Of Reaction Rate On Concentration Of Reactants Rate Enzymes Are Zero Order In A Rate Expression Why would you need a rate law? For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. V is zero order with respect to s and first order in e. We refer to these reactions as. Its differential rate law is rate = k. Enzyme. Enzymes Are Zero Order In A Rate Expression.
From biology4ibdp.weebly.com
2.5 Enzymes BIOLOGY4IBDP Enzymes Are Zero Order In A Rate Expression Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Why would you need a rate law? Its differential rate law is rate = k. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. We refer to these reactions as. Enzymes. Enzymes Are Zero Order In A Rate Expression.
From www.slideserve.com
PPT Enzyme I PowerPoint Presentation, free download ID298555 Enzymes Are Zero Order In A Rate Expression For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. Why would you need a rate law? Enzyme kinetics is. Enzymes Are Zero Order In A Rate Expression.
From derangedphysiology.com
First order, zero order and elimination Deranged Enzymes Are Zero Order In A Rate Expression We refer to these reactions as. V is zero order with respect to s and first order in e. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Its differential rate. Enzymes Are Zero Order In A Rate Expression.
From www.worthington-biochem.com
Enzyme Concentration Worthington Biochemical Enzymes Are Zero Order In A Rate Expression Why would you need a rate law? Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. We refer to these reactions as. V is zero order with respect to s and first order in e. For enzymatic reactions (or any catalytic reactions in general),. Enzymes Are Zero Order In A Rate Expression.
From facts.net
19 Mindblowing Facts About ZeroOrder Reaction Enzymes Are Zero Order In A Rate Expression Enzyme kinetics is a key aspect of biochemistry that examines the rates of. We refer to these reactions as. For enzymatic reactions (or any catalytic reactions in general), the initial rate of the reaction is proportional to [s], as it is for the uncatalyzed. V is zero order with respect to s and first order in e. Its differential rate. Enzymes Are Zero Order In A Rate Expression.
From general.chemistrysteps.com
ZeroOrder Reactions Chemistry Steps Enzymes Are Zero Order In A Rate Expression V is zero order with respect to s and first order in e. Why would you need a rate law? Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Enzymes catalyze the rate of chemical reactions by lowering the activation energy of the reaction, and they do this in a manner which is highly. For enzymatic. Enzymes Are Zero Order In A Rate Expression.