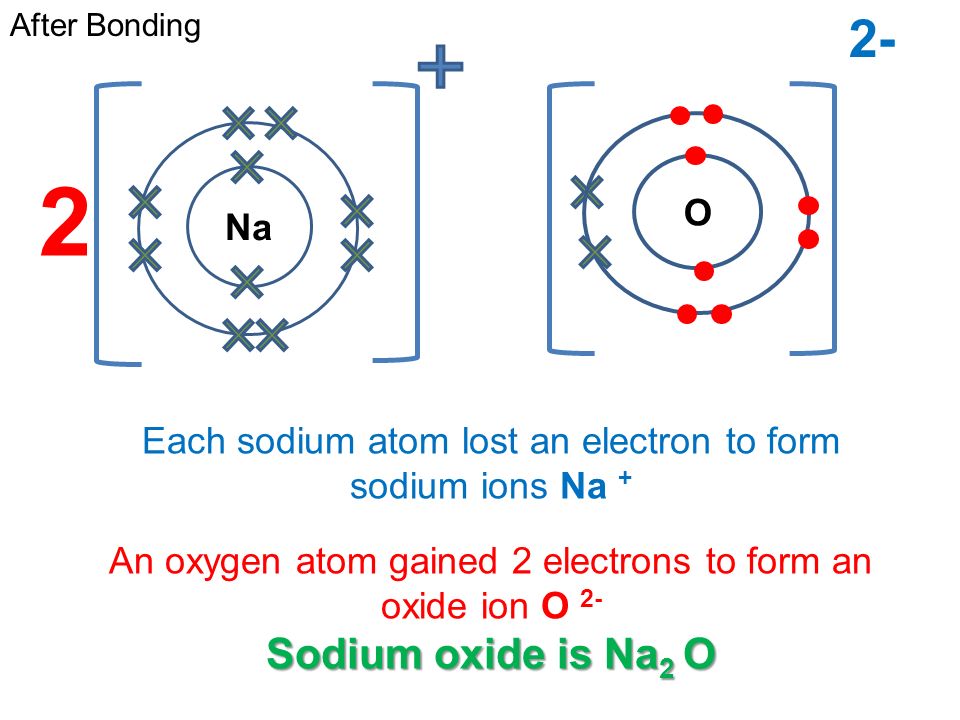
Does Sodium Form A Positive Or Negative Ion . A sodium atom loses one electron to form a sodium ion. Ionic compounds have positive ions and negative ions. Ions form when atoms lose or gain electrons. The charge on the ion can also be shown as. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. The sodium ion has an extra positive charge, shown by the + sign. When the charge is plus one, the one is left out of the chemical formula. Thus, if you commit the information in table 3.6. The chemical formula for a sodium ion is therefore na +. Ionic formulas balance the total positive and. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. All group 1 metals will form a 1+ ion.
from derekcarrsavvy-chemist.blogspot.com
The charge on the ion can also be shown as. All group 1 metals will form a 1+ ion. Thus, if you commit the information in table 3.6. The chemical formula for a sodium ion is therefore na +. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. The sodium ion has an extra positive charge, shown by the + sign. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. A sodium atom loses one electron to form a sodium ion. Ions form when atoms lose or gain electrons.
savvychemist GCSE OCR Gateway Chemistry C2.2 di Bonding and the
Does Sodium Form A Positive Or Negative Ion When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Ionic formulas balance the total positive and. The sodium ion has an extra positive charge, shown by the + sign. All group 1 metals will form a 1+ ion. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. The charge on the ion can also be shown as. Ions form when atoms lose or gain electrons. When the charge is plus one, the one is left out of the chemical formula. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Thus, if you commit the information in table 3.6. The chemical formula for a sodium ion is therefore na +. Ionic compounds have positive ions and negative ions. A sodium atom loses one electron to form a sodium ion. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Does Sodium Form A Positive Or Negative Ion The charge on the ion can also be shown as. A sodium atom loses one electron to form a sodium ion. All group 1 metals will form a 1+ ion. The sodium ion has an extra positive charge, shown by the + sign. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or. Does Sodium Form A Positive Or Negative Ion.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5413898 Does Sodium Form A Positive Or Negative Ion The charge on the ion can also be shown as. When the charge is plus one, the one is left out of the chemical formula. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. Ions form when atoms lose or gain electrons. The sodium. Does Sodium Form A Positive Or Negative Ion.
From www.pinterest.com
Na+ Electron Configuration (Sodium Ion) Electron configuration Does Sodium Form A Positive Or Negative Ion The chemical formula for a sodium ion is therefore na +. Ionic compounds have positive ions and negative ions. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. When sodium atoms form ions, they always form a 1+ charge, never a 2+. Does Sodium Form A Positive Or Negative Ion.
From alevelbiology.co.uk
Ions Types, Summary, Classification & Facts Does Sodium Form A Positive Or Negative Ion For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. The chemical formula for a sodium ion is therefore na +. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Does Sodium Form A Positive Or Negative Ion.
From www.getbodysmart.com
Cations and Anions examples and diagrams GetBodySmart Does Sodium Form A Positive Or Negative Ion The charge on the ion can also be shown as. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. The sodium ion has an extra positive charge, shown by the + sign. Thus, if you commit the information in table 3.6. Ionic formulas balance. Does Sodium Form A Positive Or Negative Ion.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Does Sodium Form A Positive Or Negative Ion The charge on the ion can also be shown as. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. The sodium ion has an extra positive charge, shown by the + sign. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its. Does Sodium Form A Positive Or Negative Ion.
From slideplayer.com
How Atoms Bond Psi ppt download Does Sodium Form A Positive Or Negative Ion The chemical formula for a sodium ion is therefore na +. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. The charge on the ion can also be shown as. Ions form when atoms lose or gain electrons. A sodium atom loses. Does Sodium Form A Positive Or Negative Ion.
From www.chemistrystudent.com
Metallic Bonding (ALevel) ChemistryStudent Does Sodium Form A Positive Or Negative Ion A sodium atom loses one electron to form a sodium ion. Ions form when atoms lose or gain electrons. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. The sodium ion has an extra positive charge, shown by the + sign. When the charge. Does Sodium Form A Positive Or Negative Ion.
From www.chemistryland.com
Lab 11 Does Sodium Form A Positive Or Negative Ion Ionic formulas balance the total positive and. Thus, if you commit the information in table 3.6. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one. Does Sodium Form A Positive Or Negative Ion.
From www.thesciencehive.co.uk
Types of Bonding* — the science sauce Does Sodium Form A Positive Or Negative Ion Ionic compounds have positive ions and negative ions. When the charge is plus one, the one is left out of the chemical formula. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. All group 1 metals will form a 1+ ion. Ionic formulas balance the total positive and. Thus,. Does Sodium Form A Positive Or Negative Ion.
From www.slideserve.com
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation, free Does Sodium Form A Positive Or Negative Ion For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. Ions form when atoms lose or gain electrons. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when.. Does Sodium Form A Positive Or Negative Ion.
From slideplayer.com
Ionic Compounds. ppt download Does Sodium Form A Positive Or Negative Ion The sodium ion has an extra positive charge, shown by the + sign. Ionic formulas balance the total positive and. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Ionic compounds have positive ions and negative ions. Thus, if you commit the. Does Sodium Form A Positive Or Negative Ion.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C2.2 di Bonding and the Does Sodium Form A Positive Or Negative Ion Thus, if you commit the information in table 3.6. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. All group 1 metals will form a 1+ ion. A cation (a positive ion) forms when a neutral atom loses one or more electrons. Does Sodium Form A Positive Or Negative Ion.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Does Sodium Form A Positive Or Negative Ion The sodium ion has an extra positive charge, shown by the + sign. A sodium atom loses one electron to form a sodium ion. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. For example, when each sodium atom in a sample. Does Sodium Form A Positive Or Negative Ion.
From slidesharenow.blogspot.com
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare Does Sodium Form A Positive Or Negative Ion When the charge is plus one, the one is left out of the chemical formula. Ions form when atoms lose or gain electrons. A sodium atom loses one electron to form a sodium ion. All group 1 metals will form a 1+ ion. Thus, if you commit the information in table 3.6. Ionic compounds have positive ions and negative ions.. Does Sodium Form A Positive Or Negative Ion.
From www.slideserve.com
PPT Chapter 2 Life ’ s Chemical Basis PowerPoint Presentation, free Does Sodium Form A Positive Or Negative Ion A sodium atom loses one electron to form a sodium ion. The sodium ion has an extra positive charge, shown by the + sign. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. For example, when each sodium atom in a sample of sodium metal (group 1) gives up. Does Sodium Form A Positive Or Negative Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Does Sodium Form A Positive Or Negative Ion Ionic formulas balance the total positive and. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. A sodium atom loses one electron to form a sodium ion. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or. Does Sodium Form A Positive Or Negative Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Does Sodium Form A Positive Or Negative Ion When the charge is plus one, the one is left out of the chemical formula. Ions form when atoms lose or gain electrons. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. A cation (a positive ion) forms when a neutral atom loses one. Does Sodium Form A Positive Or Negative Ion.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.37 Understand How Ions are formed by Electron Does Sodium Form A Positive Or Negative Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. The charge on the ion can also be shown as. All group 1 metals will form a 1+ ion. Thus, if you commit the information in table 3.6. Ionic compounds have positive ions. Does Sodium Form A Positive Or Negative Ion.
From studylibrarybeverly.z21.web.core.windows.net
How Do Positive And Negative Ions Form Does Sodium Form A Positive Or Negative Ion Ionic formulas balance the total positive and. When the charge is plus one, the one is left out of the chemical formula. Thus, if you commit the information in table 3.6. The sodium ion has an extra positive charge, shown by the + sign. Ions form when atoms lose or gain electrons. Ionic compounds have positive ions and negative ions.. Does Sodium Form A Positive Or Negative Ion.
From www.chegg.com
Solved Positive ion Negative ion Name Formula Sodium Does Sodium Form A Positive Or Negative Ion Thus, if you commit the information in table 3.6. Ionic formulas balance the total positive and. The sodium ion has an extra positive charge, shown by the + sign. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. Ionic compounds have positive ions and. Does Sodium Form A Positive Or Negative Ion.
From www.chemistrystudent.com
Ionic Bonding (ALevel) ChemistryStudent Does Sodium Form A Positive Or Negative Ion For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. Thus, if you commit the information in table 3.6. When the charge is plus one, the one is left out of the chemical formula. A cation (a positive ion) forms when a neutral atom loses. Does Sodium Form A Positive Or Negative Ion.
From www2.victoriacollege.edu
formation of ionic bonds Does Sodium Form A Positive Or Negative Ion Ions form when atoms lose or gain electrons. A sodium atom loses one electron to form a sodium ion. The sodium ion has an extra positive charge, shown by the + sign. The charge on the ion can also be shown as. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even. Does Sodium Form A Positive Or Negative Ion.
From projectopenletter.com
Do Metals Form Positive Or Negative Ions Does Sodium Form A Positive Or Negative Ion For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. A sodium atom loses one electron to form a sodium ion. Ionic compounds have positive ions and negative ions. The charge on the ion can also be shown as. When the charge is plus one,. Does Sodium Form A Positive Or Negative Ion.
From projectopenletter.com
Do Metals Form Positive Or Negative Ions Printable Form, Templates Does Sodium Form A Positive Or Negative Ion Ions form when atoms lose or gain electrons. Thus, if you commit the information in table 3.6. Ionic compounds have positive ions and negative ions. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. When the charge is plus one, the one is left out of the chemical formula.. Does Sodium Form A Positive Or Negative Ion.
From educationbunyip.z4.web.core.windows.net
Do Metals Form Positive Or Negative Ions Does Sodium Form A Positive Or Negative Ion Thus, if you commit the information in table 3.6. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. A sodium atom loses one electron to form a sodium ion. The charge on the ion can also be shown as. All group 1 metals will. Does Sodium Form A Positive Or Negative Ion.
From wou.edu
CH104 Chapter 3 Ions and Ionic Compounds Chemistry Does Sodium Form A Positive Or Negative Ion When the charge is plus one, the one is left out of the chemical formula. Ionic compounds have positive ions and negative ions. Thus, if you commit the information in table 3.6. The chemical formula for a sodium ion is therefore na +. Ions form when atoms lose or gain electrons. A cation (a positive ion) forms when a neutral. Does Sodium Form A Positive Or Negative Ion.
From www.slideserve.com
PPT Ionic Bonds (the attraction between negative ions & positive ions Does Sodium Form A Positive Or Negative Ion Ions form when atoms lose or gain electrons. The charge on the ion can also be shown as. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. The sodium ion has an extra positive charge, shown by the + sign. All group 1 metals will form a 1+ ion.. Does Sodium Form A Positive Or Negative Ion.
From periodictable.me
Sodium Electron Configuration (Na) with Orbital Diagram Does Sodium Form A Positive Or Negative Ion The sodium ion has an extra positive charge, shown by the + sign. The chemical formula for a sodium ion is therefore na +. When the charge is plus one, the one is left out of the chemical formula. Thus, if you commit the information in table 3.6. When sodium atoms form ions, they always form a 1+ charge, never. Does Sodium Form A Positive Or Negative Ion.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Does Sodium Form A Positive Or Negative Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. Ionic formulas balance the total positive and. A. Does Sodium Form A Positive Or Negative Ion.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind Does Sodium Form A Positive Or Negative Ion Thus, if you commit the information in table 3.6. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. A sodium atom loses one electron to form a sodium ion. The chemical formula for a sodium ion is therefore na +. A cation (a positive ion) forms when a neutral. Does Sodium Form A Positive Or Negative Ion.
From studydemandants.z21.web.core.windows.net
Do Metals Form Positive Or Negative Ions Does Sodium Form A Positive Or Negative Ion Ionic compounds have positive ions and negative ions. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. The charge on the ion can also be shown as. A cation (a positive ion) forms when a neutral atom loses one or more electrons. Does Sodium Form A Positive Or Negative Ion.
From slideplayer.com
WarmUp Describe the difference between ionic and covalent bonds Does Sodium Form A Positive Or Negative Ion For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. Ionic compounds have positive ions and negative ions. Ions form when atoms lose or gain electrons. A sodium atom loses one electron to form a sodium ion. All group 1 metals will form a 1+. Does Sodium Form A Positive Or Negative Ion.
From brainly.com
Sodium hydroxide completely dissociates into positive and negative ions Does Sodium Form A Positive Or Negative Ion When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Ionic formulas balance the total positive and. The chemical formula for a sodium ion is therefore na +. A sodium atom loses one electron to form a sodium ion. Ions form when atoms lose or gain electrons. The sodium ion. Does Sodium Form A Positive Or Negative Ion.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Does Sodium Form A Positive Or Negative Ion When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. The chemical formula for a sodium ion is therefore na +. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. A cation (a positive. Does Sodium Form A Positive Or Negative Ion.