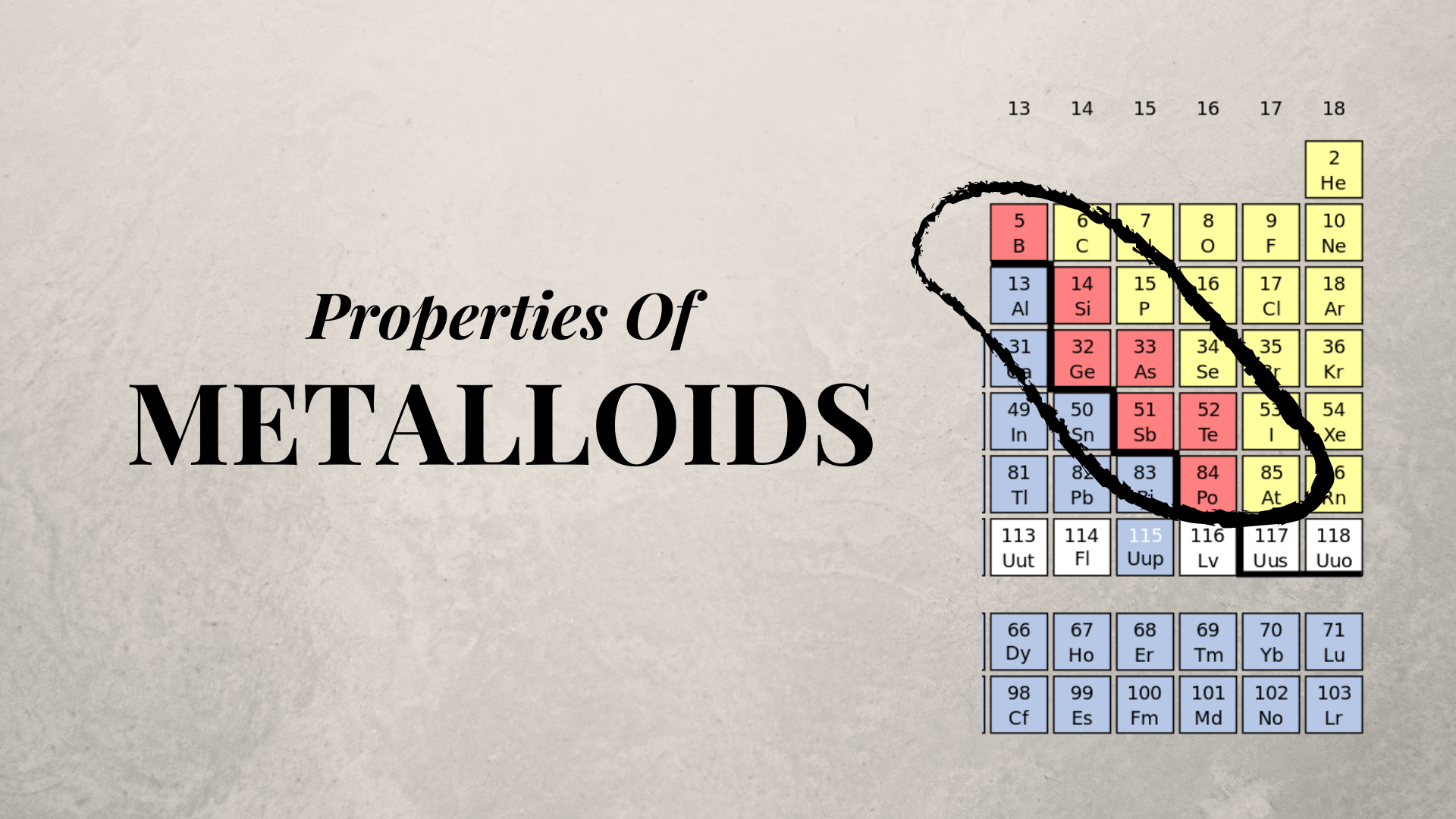
Are Metalloids Transition Metals . Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Most of the elements are metals. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Here’s a look at the different ways of. From left to right in the periodic table, these categories include the highly reactive alkali metals; Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals.
from sciencetrends.com
Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Here’s a look at the different ways of. Most of the elements are metals. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. From left to right in the periodic table, these categories include the highly reactive alkali metals;
4 Properties Of Metalloids Science Trends
Are Metalloids Transition Metals Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. From left to right in the periodic table, these categories include the highly reactive alkali metals; Most of the elements are metals. Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Here’s a look at the different ways of.
From utedzz.blogspot.com
Periodic Table With Metals Metalloids And Nonmetals Labeled Periodic Are Metalloids Transition Metals Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. From left to right in the periodic table, these categories include the highly reactive alkali metals; Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that. Are Metalloids Transition Metals.
From slidetodoc.com
The Periodic Table metals metalloids nonmetals semimetals transition Are Metalloids Transition Metals Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Most of the elements are metals. Here’s a look at the different ways of. Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Metals include the. Are Metalloids Transition Metals.
From www.chemistrylearner.com
Metalloids Chemistry Learner Are Metalloids Transition Metals Most of the elements are metals. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Here’s a look at the different ways of. Metalloids and transition metals are two distinct groups of elements with their own set of. Are Metalloids Transition Metals.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Are Metalloids Transition Metals Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Here’s a look at the different ways of. Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Most of the elements are metals. Metals. Are Metalloids Transition Metals.
From www.priyamstudycentre.com
Transition Metals Elements, Definition, List, Properties Are Metalloids Transition Metals Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Here’s a look at the different ways of. Most of the elements are metals. From left to right in the periodic table, these categories include the. Are Metalloids Transition Metals.
From www.slideserve.com
PPT Metals, Metalloids, and Nonmetals PowerPoint Presentation, free Are Metalloids Transition Metals Most of the elements are metals. Here’s a look at the different ways of. Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Metals include the. Are Metalloids Transition Metals.
From www.slideserve.com
PPT Principles of Chemistry, Chapt . 2 Atomic Structure and The Are Metalloids Transition Metals Most of the elements are metals. From left to right in the periodic table, these categories include the highly reactive alkali metals; Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Here’s a look at the different ways of. Moving from left to right across the periodic table, an electron is. Are Metalloids Transition Metals.
From utedzz.blogspot.com
Periodic Table Showing Metals Metalloids And Nonmetals Periodic Table Are Metalloids Transition Metals Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Most of the elements are metals. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. From left to right in the periodic table, these categories include the highly reactive alkali metals; Metals include the alkali metal, alkaline. Are Metalloids Transition Metals.
From www.differencebetween.com
Difference Between Metals and Metalloids Compare the Difference Are Metalloids Transition Metals From left to right in the periodic table, these categories include the highly reactive alkali metals; Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Most of the elements are metals. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Transition metals are defined. Are Metalloids Transition Metals.
From www.researchgate.net
(PDF) Metals Typical and Less Typical, Transition and Inner Transition Are Metalloids Transition Metals Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Here’s a look at the different ways of. Most of the elements are metals. Metalloids and transition metals are two distinct groups of elements with their own set of. Are Metalloids Transition Metals.
From www.chegg.com
Solved The Periodic Table of Elements He Transition Metals Are Metalloids Transition Metals Most of the elements are metals. Here’s a look at the different ways of. Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Moving from left to right across the periodic table, an electron is. Are Metalloids Transition Metals.
From www.slideserve.com
PPT The Modern Periodic Table Chapter 6 PowerPoint Presentation, free Are Metalloids Transition Metals Here’s a look at the different ways of. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Moving from left to right across the periodic table, an electron is added to the d orbital of. Are Metalloids Transition Metals.
From periodictableguide.com
Different Types of Metals on the Periodic table (With Image) Are Metalloids Transition Metals Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Here’s a look at the different ways of. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Most of the elements are metals. From left to right in the periodic table, these categories include the highly reactive alkali. Are Metalloids Transition Metals.
From newtondesk.com
Metalloids (Periodic Table) Properties, Uses, & Facts NewtonDesk Are Metalloids Transition Metals Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Most of the elements are metals. Here’s a look at the. Are Metalloids Transition Metals.
From courses.lumenlearning.com
Occurrence, Preparation, and Properties of Transition Metals and Their Are Metalloids Transition Metals From left to right in the periodic table, these categories include the highly reactive alkali metals; Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Most of the elements are metals. Here’s a look at. Are Metalloids Transition Metals.
From www.breakingatom.com
Metalloid Definition Are Metalloids Transition Metals Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. From left to right in the periodic table, these categories include the highly reactive alkali metals; Here’s a look at the different ways of. Moving from. Are Metalloids Transition Metals.
From www.slideserve.com
PPT Transition Metals PowerPoint Presentation, free download ID2275684 Are Metalloids Transition Metals From left to right in the periodic table, these categories include the highly reactive alkali metals; Here’s a look at the different ways of. Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Metals include the alkali metal, alkaline earth, transition metal,. Are Metalloids Transition Metals.
From www.pinterest.com
the periodic table is shown in this graphic Are Metalloids Transition Metals Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Most of the elements are metals. From left to right in the periodic table, these categories include the highly reactive alkali metals; Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Transition metal, any of various chemical. Are Metalloids Transition Metals.
From www.differencebetween.com
Difference Between Transition Metals and Metalloids Compare the Are Metalloids Transition Metals Here’s a look at the different ways of. Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Transition metal, any of various chemical elements that have. Are Metalloids Transition Metals.
From sciencenotes.org
List of Metalloids or Semimetals Are Metalloids Transition Metals Here’s a look at the different ways of. Most of the elements are metals. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Transition metals are defined as those elements that have (or readily form) partially. Are Metalloids Transition Metals.
From newtondesk.com
Metalloids (Periodic Table) Properties, Uses, & Facts Are Metalloids Transition Metals Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Most of the elements are metals. From left to right in the periodic table, these categories include the highly reactive alkali metals; Here’s a look at the different ways of. Metalloids and transition. Are Metalloids Transition Metals.
From www.slideserve.com
PPT Chapter 3 Elements, Compounds, and the Periodic Table PowerPoint Are Metalloids Transition Metals Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Most of the elements are metals. Moving from left to right across the. Are Metalloids Transition Metals.
From periodictableguide.com
Where are Transition Metals located on the Periodic Table? Are Metalloids Transition Metals Metalloids and transition metals are two distinct groups of elements with their own set of attributes. From left to right in the periodic table, these categories include the highly reactive alkali metals; Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Transition. Are Metalloids Transition Metals.
From utedzz.blogspot.com
Labeled Periodic Table Inner Transition Metals Periodic Table Timeline Are Metalloids Transition Metals Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Here’s a look at the different ways of. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Transition metal, any of various. Are Metalloids Transition Metals.
From www.youtube.com
Properties of the TRANSITION METALS Periodic Table YouTube Are Metalloids Transition Metals Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Most of the elements are metals. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Here’s a look at the different ways of. Transition metal, any. Are Metalloids Transition Metals.
From www.slideserve.com
PPT ELEMENT CLASSES PowerPoint Presentation, free download ID149914 Are Metalloids Transition Metals Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Metals. Are Metalloids Transition Metals.
From www.youtube.com
The Transition Metals YouTube Are Metalloids Transition Metals Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups.. Are Metalloids Transition Metals.
From www.adda247.com
What are metalloids? Definition, Properties and Example Are Metalloids Transition Metals Here’s a look at the different ways of. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Most of the elements are metals. Metalloids and transition. Are Metalloids Transition Metals.
From www.slideserve.com
PPT The Periodic Table! PowerPoint Presentation, free download ID Are Metalloids Transition Metals Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. From left to right in the periodic table, these categories include the highly reactive alkali metals; Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Most of the elements are metals. Moving from left to. Are Metalloids Transition Metals.
From www.coursehero.com
Periodicity General Chemistry I Course Hero Are Metalloids Transition Metals Here’s a look at the different ways of. Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. From left to right in the periodic table, these categories include the highly reactive alkali metals; Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Most of. Are Metalloids Transition Metals.
From sciencetrends.com
4 Properties Of Metalloids Science Trends Are Metalloids Transition Metals Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Here’s a look at the different ways of. From left to right in the periodic table, these categories include the highly reactive alkali metals; Metals include the alkali metal, alkaline earth, transition metal,. Are Metalloids Transition Metals.
From www.slideserve.com
PPT Naming Chemicals PowerPoint Presentation, free download ID584942 Are Metalloids Transition Metals From left to right in the periodic table, these categories include the highly reactive alkali metals; Metalloids and transition metals are two distinct groups of elements with their own set of attributes. Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Metals. Are Metalloids Transition Metals.
From truyenhinhcapsongthu.net
Where Are Metals Located On The Periodic Table (With Images) Are Metalloids Transition Metals Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. From left to right in the periodic table, these categories include the highly reactive alkali metals; Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that. Are Metalloids Transition Metals.
From sciencenotes.org
Metalloids Science Notes and Projects Are Metalloids Transition Metals Transition metal, any of various chemical elements that have valence electrons—i.e., electrons that can participate in the formation. Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Metals include the alkali metal, alkaline earth, transition metal, basic metal, lanthanide, and actinide groups. Most of the elements are metals. Metalloids and transition metals are. Are Metalloids Transition Metals.
From www.sliderbase.com
Element Classes Presentation Chemistry Are Metalloids Transition Metals Moving from left to right across the periodic table, an electron is added to the d orbital of each atom, transitioning from group 2 to group 13. Most of the elements are metals. Metalloids and transition metals are two distinct groups of elements with their own set of attributes. From left to right in the periodic table, these categories include. Are Metalloids Transition Metals.