How To Find A Final Temperature In Calorimetry . The calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. Calculating the final temperature in calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. In calorimetry, the final temperature can be calculated using the formula: Additionally, it can find the. Assuming that all heat transfer occurs between the copper and the water, calculate the final.
from www.slideshare.net
Apply the first law of thermodynamics to calorimetry. This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. Calculating the final temperature in calorimetry. It can analyze the heat exchange between up to 3 objects. In calorimetry, the final temperature can be calculated using the formula: Additionally, it can find the. The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents.
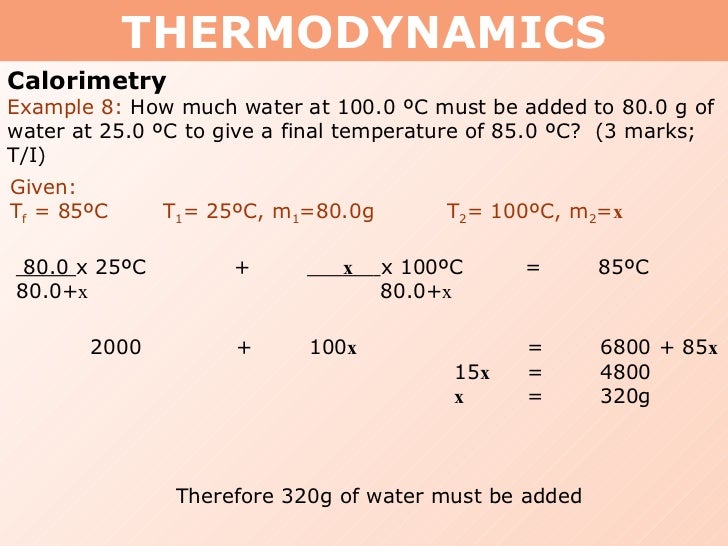
Tang 01 heat capacity and calorimetry
How To Find A Final Temperature In Calorimetry Apply the first law of thermodynamics to calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. Additionally, it can find the. Calculating the final temperature in calorimetry. In calorimetry, the final temperature can be calculated using the formula: With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. It can analyze the heat exchange between up to 3 objects. The calorimetry calculator can help you solve complex calorimetry problems. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. Assuming that all heat transfer occurs between the copper and the water, calculate the final.
From www.youtube.com
Solving for Final Temperature in Calorimetry YouTube How To Find A Final Temperature In Calorimetry Assuming that all heat transfer occurs between the copper and the water, calculate the final. Calculating the final temperature in calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. This chemistry. How To Find A Final Temperature In Calorimetry.
From goodttorials.blogspot.com
How To Find Final Temperature Without Specific Heat How To Find A Final Temperature In Calorimetry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. It can analyze the heat exchange between up to 3 objects. This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. In calorimetry, the final temperature can be calculated using the formula: With a calorimeter,. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Determine the final Temperature of the room Thermodynamics YouTube How To Find A Final Temperature In Calorimetry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It can analyze the heat exchange between up to. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Calculating final temperature through heat exchange in thermal How To Find A Final Temperature In Calorimetry Additionally, it can find the. This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. It can analyze the heat exchange between up to 3 objects. Compare heat flow from hot to cold objects. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
How to Calculate Specific Heat (Thermochemistry) YouTube How To Find A Final Temperature In Calorimetry Assuming that all heat transfer occurs between the copper and the water, calculate the final. In calorimetry, the final temperature can be calculated using the formula: It can analyze the heat exchange between up to 3 objects. Additionally, it can find the. The calorimetry problems can be used to find the initial or the final temperature of the substances when. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Calorimetry Lecture 4 Thermal Physics Explanation of Temperature Time How To Find A Final Temperature In Calorimetry It can analyze the heat exchange between up to 3 objects. The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. Apply the first law of thermodynamics to calorimetry. With. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Thermochemistry How to Calculate Final Temperature YouTube How To Find A Final Temperature In Calorimetry The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. Calculating the final temperature in calorimetry. Apply the first law of thermodynamics to calorimetry. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. In calorimetry, the final. How To Find A Final Temperature In Calorimetry.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment How To Find A Final Temperature In Calorimetry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calculating the final temperature in calorimetry. Additionally, it can find the. The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. This chemistry video tutorial explains how to find. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube How To Find A Final Temperature In Calorimetry This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. Assuming that all heat transfer occurs between the copper and the water, calculate the final. Calculating the final temperature in calorimetry. Additionally, it can find the. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Temperature and Heat Calorimetry. Level 2, Example 2 YouTube How To Find A Final Temperature In Calorimetry The calorimetry calculator can help you solve complex calorimetry problems. This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. Calculating the final temperature in calorimetry. It can analyze the heat exchange between up to 3 objects. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in. How To Find A Final Temperature In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6133898 How To Find A Final Temperature In Calorimetry This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. It can analyze the heat exchange between up to 3 objects. Apply the first law of thermodynamics to calorimetry. Calculating the final temperature in calorimetry. The calorimetry problems can be used to find the initial or the final temperature of the substances when. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube How To Find A Final Temperature In Calorimetry This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. Apply the first law of thermodynamics to calorimetry. In calorimetry, the final temperature can be calculated using the formula: It. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Charles's Law Solving for Final Temperature YouTube How To Find A Final Temperature In Calorimetry Apply the first law of thermodynamics to calorimetry. Assuming that all heat transfer occurs between the copper and the water, calculate the final. This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in. How To Find A Final Temperature In Calorimetry.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent How To Find A Final Temperature In Calorimetry In calorimetry, the final temperature can be calculated using the formula: It can analyze the heat exchange between up to 3 objects. The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. Assuming that all heat transfer occurs between the copper and the water, calculate the final.. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
What is the Final Temperature given Heat (q=mcΔT) YouTube How To Find A Final Temperature In Calorimetry Apply the first law of thermodynamics to calorimetry. The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The calorimetry calculator can help you solve complex calorimetry problems. Calculate heat, temperature. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Specific Heat Solving for the Final Temperature YouTube How To Find A Final Temperature In Calorimetry The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. It can analyze the heat exchange between up to 3 objects. Compare heat flow from hot to cold objects. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Calorimetry calculation YouTube How To Find A Final Temperature In Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. In calorimetry, the final temperature can be calculated using the formula: Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Apply the first law of thermodynamics to calorimetry. Additionally, it can find the. The calorimetry. How To Find A Final Temperature In Calorimetry.
From www.tec-science.com
Final temperature of mixtures (Richmann’s law) tecscience How To Find A Final Temperature In Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Assuming that all heat transfer occurs between the copper and the water, calculate the final. Additionally, it can find the. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. It can analyze the heat exchange. How To Find A Final Temperature In Calorimetry.
From www.showme.com
4 steps for solving calorimetry problems Science, Chemistry ShowMe How To Find A Final Temperature In Calorimetry It can analyze the heat exchange between up to 3 objects. Calculating the final temperature in calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Apply the first law of thermodynamics to calorimetry. The calorimetry problems can be used to find the initial or the final temperature of the substances. How To Find A Final Temperature In Calorimetry.
From www.pinterest.com
Calorimetry Using q=mΔTc to find Temperature + Example YouTube How To Find A Final Temperature In Calorimetry Apply the first law of thermodynamics to calorimetry. The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. It can analyze the heat exchange between up to 3 objects. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter.. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Finding the Equilibrium Temperature of a Mixture of Different How To Find A Final Temperature In Calorimetry Calculating the final temperature in calorimetry. The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. In calorimetry, the final temperature can be. How To Find A Final Temperature In Calorimetry.
From quiztricksters.z21.web.core.windows.net
How To Calculate Calorimeter How To Find A Final Temperature In Calorimetry The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. It can analyze the heat exchange between up to 3 objects. This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. Apply the first law of thermodynamics to calorimetry. Assuming. How To Find A Final Temperature In Calorimetry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How To Find A Final Temperature In Calorimetry Calculating the final temperature in calorimetry. The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. Additionally, it can find the. In calorimetry, the final temperature can be calculated using the formula: Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Physics Final temperature of mixture Thermal properties of matter How To Find A Final Temperature In Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. Additionally, it can find the. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. This chemistry video tutorial explains how to find the final temperature in. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube How To Find A Final Temperature In Calorimetry The calorimetry calculator can help you solve complex calorimetry problems. Assuming that all heat transfer occurs between the copper and the water, calculate the final. In calorimetry, the final temperature can be calculated using the formula: Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Principle of Calorimetry YouTube How To Find A Final Temperature In Calorimetry The calorimetry calculator can help you solve complex calorimetry problems. Assuming that all heat transfer occurs between the copper and the water, calculate the final. In calorimetry, the final temperature can be calculated using the formula: Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculate heat, temperature change, and specific heat after. How To Find A Final Temperature In Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 How To Find A Final Temperature In Calorimetry The calorimetry calculator can help you solve complex calorimetry problems. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Assuming that all heat transfer occurs between the copper and the water, calculate the final. It can analyze the heat exchange between up to 3 objects. Additionally, it can find the. In calorimetry, the. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
How to find calorimeter constant YouTube How To Find A Final Temperature In Calorimetry Apply the first law of thermodynamics to calorimetry. This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. Additionally, it can find the. In calorimetry, the final temperature can be calculated using the formula: It can analyze the heat exchange between up to 3 objects. Assuming that all heat transfer occurs between the. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Heat Thermal Equilibrium Finding Final Temp YouTube How To Find A Final Temperature In Calorimetry The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. Additionally, it can find the. In calorimetry, the final temperature can be calculated using the formula: Calculating the final temperature in calorimetry. Assuming that all heat transfer occurs between the copper and the water, calculate the final.. How To Find A Final Temperature In Calorimetry.
From www.slideshare.net
Tang 01 heat capacity and calorimetry How To Find A Final Temperature In Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. Additionally, it can find the. The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Coffee Cup Calorimeter Calculate Enthalpy Change, Constant Pressure How To Find A Final Temperature In Calorimetry Assuming that all heat transfer occurs between the copper and the water, calculate the final. This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. In calorimetry, the final temperature can be calculated using the formula: The calorimetry calculator can help you solve complex calorimetry problems. With a calorimeter, you can measure reaction. How To Find A Final Temperature In Calorimetry.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter How To Find A Final Temperature In Calorimetry Assuming that all heat transfer occurs between the copper and the water, calculate the final. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calculating the final temperature in calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It can analyze the heat. How To Find A Final Temperature In Calorimetry.
From www.gbu-presnenskij.ru
Chapter 25 Essential Calorimetry Formulae James Kennedy, 47 OFF How To Find A Final Temperature In Calorimetry It can analyze the heat exchange between up to 3 objects. Apply the first law of thermodynamics to calorimetry. Additionally, it can find the. This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The. How To Find A Final Temperature In Calorimetry.
From www.youtube.com
Unit 3 Calculating final Temperature YouTube How To Find A Final Temperature In Calorimetry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. Calculating the final temperature in calorimetry. This chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems.. How To Find A Final Temperature In Calorimetry.
From www.slideshare.net
Tang 01 heat capacity and calorimetry How To Find A Final Temperature In Calorimetry Calculating the final temperature in calorimetry. The calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a chemical reaction. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Apply the first law of thermodynamics to calorimetry. It can analyze the heat. How To Find A Final Temperature In Calorimetry.