Titration Curve Of Phosphoric Acid . A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Titrations of polyprotic acids have more than. K a corresponds to the reaction of a weak acid with. As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example.
from www.chegg.com
A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. Titrations of polyprotic acids have more than. Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. K a corresponds to the reaction of a weak acid with. As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example.
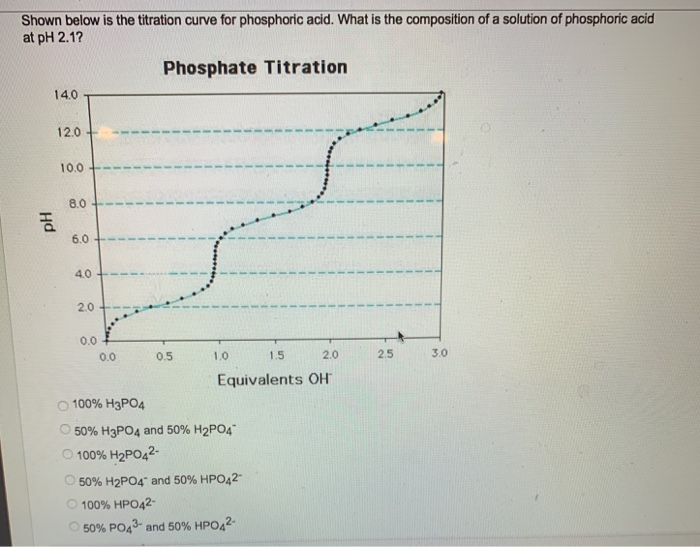
Solved Shown below is the titration curve for phosphoric
Titration Curve Of Phosphoric Acid Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. K a corresponds to the reaction of a weak acid with. As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. Titrations of polyprotic acids have more than. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid.
From www.scribd.com
Phosphoric Acid Titration PDF Titration Chemistry Titration Curve Of Phosphoric Acid In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. K a corresponds to the reaction of a weak acid with. Titrations of polyprotic acids have more than. Sketch out a. Titration Curve Of Phosphoric Acid.
From oneclass.com
OneClass Consider the titration curve of phosphoric acid (H3PO4) shown Titration Curve Of Phosphoric Acid As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. A graph of ph versus added titrant is called a titration curve, and. Titration Curve Of Phosphoric Acid.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titration Curve Of Phosphoric Acid Titrations of polyprotic acids have more than. As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. K a corresponds to the reaction of a weak acid with. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. Sketch out a. Titration Curve Of Phosphoric Acid.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Of Phosphoric Acid As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. In the first case acid has to be titrated against indicator changing color around. Titration Curve Of Phosphoric Acid.
From saylordotorg.github.io
AcidBase Titrations Titration Curve Of Phosphoric Acid Titrations of polyprotic acids have more than. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. As an acid, a polyprotic acids have a very. Titration Curve Of Phosphoric Acid.
From www.vrogue.co
Figure 10 11 Strong Acid And Weak Base Titration Curv vrogue.co Titration Curve Of Phosphoric Acid Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. A graph of ph versus added titrant is called a titration curve, and. Titration Curve Of Phosphoric Acid.
From www.numerade.com
SOLVED The titration curve shown below is for the titration of Titration Curve Of Phosphoric Acid K a corresponds to the reaction of a weak acid with. Titrations of polyprotic acids have more than. Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In. Titration Curve Of Phosphoric Acid.
From www.youtube.com
Amino Acids Titration Curves Explained YouTube Titration Curve Of Phosphoric Acid As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. K a corresponds to the reaction of a weak acid with. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. A graph of ph versus added titrant is called a. Titration Curve Of Phosphoric Acid.
From hicensvanderkruijs.blogspot.com
The Graph Shows The Titration Curves Of A 1M Solution / Consider The Titration Curve Of Phosphoric Acid Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. K a corresponds to the reaction of a weak acid with. As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. A graph of ph versus added titrant is called a titration curve, and. Titration Curve Of Phosphoric Acid.
From www.scribd.com
Phosphoric Acid Titration Curve PDF Titration Curve Of Phosphoric Acid Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of. Titration Curve Of Phosphoric Acid.
From www.chegg.com
A titration curve for the titration of phosphoric Titration Curve Of Phosphoric Acid Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures. Titration Curve Of Phosphoric Acid.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Titration Curve Of Phosphoric Acid Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. In the first case acid has to be titrated. Titration Curve Of Phosphoric Acid.
From www.coursehero.com
[Solved] What makes phosphoric acid's titration curve different from Titration Curve Of Phosphoric Acid A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. Titrations of polyprotic acids have more than. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. As an acid, a polyprotic acids have a very small. Titration Curve Of Phosphoric Acid.
From www.chegg.com
Solved Shown below is the titration curve for phosphoric Titration Curve Of Phosphoric Acid In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. Titrations of polyprotic acids have more than. K a corresponds to the reaction of a weak acid with. Phosphoric acid can. Titration Curve Of Phosphoric Acid.
From www.researchgate.net
The acidbase titration curve plotted against the difference in pH and Titration Curve Of Phosphoric Acid A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. Sketch out a plot representing the titration of a weak monoprotic acid by a. Titration Curve Of Phosphoric Acid.
From www.chegg.com
Solved (6) Given the titration curve below for phosphoric Titration Curve Of Phosphoric Acid A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak. Titration Curve Of Phosphoric Acid.
From www.chemicals.co.uk
How Is Phosphoric Acid Made? The Chemistry Blog Titration Curve Of Phosphoric Acid A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. As an acid, a polyprotic acids have a very. Titration Curve Of Phosphoric Acid.
From ar.inspiredpencil.com
H3po4 Titration Curve Titration Curve Of Phosphoric Acid In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. K a corresponds to the reaction of a weak acid with. Titrations of polyprotic acids have more than. Sketch out a plot representing the titration of a. Titration Curve Of Phosphoric Acid.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Titration Curve Of Phosphoric Acid As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. Titrations of polyprotic acids have more than. K a corresponds to the reaction of a weak acid with. A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically. Titration Curve Of Phosphoric Acid.
From chart-studio.plotly.com
Phosphoric Acid Titration Curve line chart made by Sanguimora plotly Titration Curve Of Phosphoric Acid Titrations of polyprotic acids have more than. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. K a corresponds to the reaction of a weak acid. Titration Curve Of Phosphoric Acid.
From www.coursehero.com
[Solved] What makes phosphoric acid's titration curve different from Titration Curve Of Phosphoric Acid K a corresponds to the reaction of a weak acid with. As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. A graph of ph versus added titrant is called a titration curve, and. Titration Curve Of Phosphoric Acid.
From www.researchgate.net
Potentiometric titration curves and first derivates of native and Titration Curve Of Phosphoric Acid In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. K a corresponds to the reaction of a weak acid with. A graph of ph versus. Titration Curve Of Phosphoric Acid.
From mungfali.com
Phosphoric Acid Titration Curve Titration Curve Of Phosphoric Acid A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. Sketch out. Titration Curve Of Phosphoric Acid.
From ar.inspiredpencil.com
H3po4 Titration Curve Titration Curve Of Phosphoric Acid K a corresponds to the reaction of a weak acid with. As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. In the first. Titration Curve Of Phosphoric Acid.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Curve Of Phosphoric Acid K a corresponds to the reaction of a weak acid with. Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In the first case acid has to be. Titration Curve Of Phosphoric Acid.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Of Phosphoric Acid K a corresponds to the reaction of a weak acid with. Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. A graph of ph versus added titrant is called a titration curve, and the point at. Titration Curve Of Phosphoric Acid.
From www.youtube.com
REPRESENTING THE TITRATION CURVE OF PHOSPHORIC ACID YouTube Titration Curve Of Phosphoric Acid As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. K a corresponds to the reaction of a weak acid with. A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. Sketch out a. Titration Curve Of Phosphoric Acid.
From www.youtube.com
Phosphoric acid titration curve YouTube Titration Curve Of Phosphoric Acid K a corresponds to the reaction of a weak acid with. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. Titrations of. Titration Curve Of Phosphoric Acid.
From www.youtube.com
AP Lecture Phosphoric Acid Titration YouTube Titration Curve Of Phosphoric Acid Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. K a corresponds to the reaction of a weak acid with. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. A graph of ph versus. Titration Curve Of Phosphoric Acid.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curve Of Phosphoric Acid Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. K a corresponds to the reaction of a weak acid with. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. Titrations of polyprotic acids have more than. As an acid, a polyprotic acids have a very. Titration Curve Of Phosphoric Acid.
From www.chegg.com
Solved Shown below is the titration curve for phosphoric Titration Curve Of Phosphoric Acid As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a. Titration Curve Of Phosphoric Acid.
From saylordotorg.github.io
AcidBase Titrations Titration Curve Of Phosphoric Acid K a corresponds to the reaction of a weak acid with. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. Titrations of polyprotic acids have more. Titration Curve Of Phosphoric Acid.
From www.chegg.com
Solved Shown below is the titration curve for phosphoric Titration Curve Of Phosphoric Acid Titrations of polyprotic acids have more than. A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. As an acid, a polyprotic acids have a very small acid dissociation constant. Titration Curve Of Phosphoric Acid.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Of Phosphoric Acid As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. Titrations of polyprotic acids have more than. In the first case acid has to be titrated against indicator changing color around ph 4.7 (for example. Phosphoric acid can be titrated either as a monoprotic acid or as a diprotic. Titration Curve Of Phosphoric Acid.
From www.studocu.com
Experiment 6 p H Titration of Phosphoric Acid 0. 0. 6. 8. 10. 12. 14 Titration Curve Of Phosphoric Acid As an acid, a polyprotic acids have a very small acid dissociation constant (\(k_a\)), which measures the strength of the acid. A graph of ph versus added titrant is called a titration curve, and the point at which the ph changes drastically is called the equivalence. In the first case acid has to be titrated against indicator changing color around. Titration Curve Of Phosphoric Acid.