Zinc Copper Ii Nitrate Balanced Equation . The balanced equation will be calculated along with the. In order to balance zn on both sides we: The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. 1 atom in reagents and 2 atoms in products. Multiply coefficient for zn by 2 2 zn +. The numbers of n and o atoms on either side of the equation are now equal, and so the equation is balanced. Zn(s) + cu(n o3)2(aq) → zn(n o3)2(aq) + cu(s) explanation: Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will appear. The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. Check your learning write a. Zinc is a more active metal than copper (it lies higher up the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of.
from www.numerade.com
The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. The numbers of n and o atoms on either side of the equation are now equal, and so the equation is balanced. In order to balance zn on both sides we: The balanced equation will appear. Zn(s) + cu(n o3)2(aq) → zn(n o3)2(aq) + cu(s) explanation: The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. 1 atom in reagents and 2 atoms in products. Multiply coefficient for zn by 2 2 zn +. $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of.
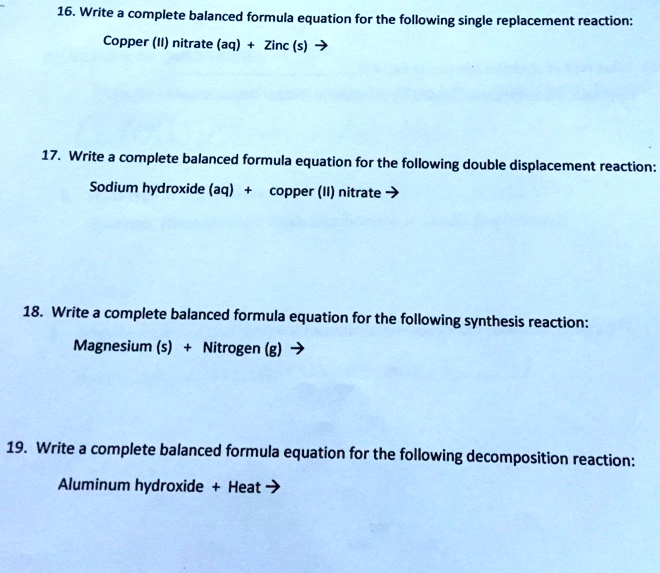
SOLVED 16. Write complete balanced formula equation for the following
Zinc Copper Ii Nitrate Balanced Equation Zn(s) + cu(n o3)2(aq) → zn(n o3)2(aq) + cu(s) explanation: Zn(s) + cu(n o3)2(aq) → zn(n o3)2(aq) + cu(s) explanation: 1 atom in reagents and 2 atoms in products. $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. Enter an equation of an ionic chemical equation and press the balance button. The numbers of n and o atoms on either side of the equation are now equal, and so the equation is balanced. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Check your learning write a. In order to balance zn on both sides we: Zinc is a more active metal than copper (it lies higher up the. The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. The balanced equation will be calculated along with the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Multiply coefficient for zn by 2 2 zn +. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. The balanced equation will appear.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Zinc Copper Ii Nitrate Balanced Equation The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. The numbers of n and o atoms on either side of the equation are now equal, and so the equation is balanced. In order to balance zn on both sides we: Zn(s) + cu(n o3)2(aq) → zn(n o3)2(aq) + cu(s) explanation: To balance a chemical. Zinc Copper Ii Nitrate Balanced Equation.
From www.slideshare.net
Tang 02 balancing redox reactions 2 Zinc Copper Ii Nitrate Balanced Equation Zn(s) + cu(n o3)2(aq) → zn(n o3)2(aq) + cu(s) explanation: The balanced equation will be calculated along with the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. The numbers of n and o atoms on either side of the equation are now equal, and. Zinc Copper Ii Nitrate Balanced Equation.
From www.coursehero.com
[Solved] When zinc metal is placed into copper(II) nitrate solution, a Zinc Copper Ii Nitrate Balanced Equation The balanced equation will be calculated along with the. Check your learning write a. 1 atom in reagents and 2 atoms in products. The numbers of n and o atoms on either side of the equation are now equal, and so the equation is balanced. Multiply coefficient for zn by 2 2 zn +. Zinc is a more active metal. Zinc Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED balanced equation; Solutions of magnesium iodide and copper (II Zinc Copper Ii Nitrate Balanced Equation $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. In order to balance zn on both sides we: The balanced equation will appear. 1 atom in reagents and 2 atoms in products. Multiply coefficient for zn by 2 2 zn +. Zinc is a more active metal than copper (it lies higher up the. Enter an equation of an ionic chemical equation and. Zinc Copper Ii Nitrate Balanced Equation.
From www.chegg.com
Solved Reactants Observations Zinc +copper(II) sulfate Zinc Copper Ii Nitrate Balanced Equation $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Multiply coefficient for zn by 2 2 zn +. The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. Check your learning write a. The equation above indicates that one mole of. Zinc Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED Copper (II) nitrate and sodium hydrogen carbonate react Zinc Copper Ii Nitrate Balanced Equation Zn(s) + cu(n o3)2(aq) → zn(n o3)2(aq) + cu(s) explanation: $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. Check your learning write a. Zinc is a more active metal than. Zinc Copper Ii Nitrate Balanced Equation.
From www.toppr.com
Identify the type of reactions taking place in each of the following Zinc Copper Ii Nitrate Balanced Equation The balanced equation will be calculated along with the. The numbers of n and o atoms on either side of the equation are now equal, and so the equation is balanced. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. Multiply coefficient for zn by 2 2. Zinc Copper Ii Nitrate Balanced Equation.
From www.toppr.com
Write balanced chemical equations for the followin Zinc Copper Ii Nitrate Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Enter an equation of an ionic chemical equation and press the balance button. Zinc is a more active metal than copper (it lies higher up the. The numbers of n and o atoms on either side of the equation are now equal, and. Zinc Copper Ii Nitrate Balanced Equation.
From mungfali.com
Solved 1. Aqueous Ammonia Solution And Copper (ii) Nitrate 306 Zinc Copper Ii Nitrate Balanced Equation Multiply coefficient for zn by 2 2 zn +. The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Check your learning write a. In order to balance zn on both. Zinc Copper Ii Nitrate Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + Cu(NO3)2 = Cu + Zn(NO3)2 Zinc Copper Ii Nitrate Balanced Equation The balanced equation will appear. Check your learning write a. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zinc is a more active metal than copper (it lies higher up the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to. Zinc Copper Ii Nitrate Balanced Equation.
From www.slideserve.com
PPT Ch. 8 Chemical Equations and Reactions PowerPoint Presentation Zinc Copper Ii Nitrate Balanced Equation The balanced equation will appear. The numbers of n and o atoms on either side of the equation are now equal, and so the equation is balanced. In order to balance zn on both sides we: The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of.. Zinc Copper Ii Nitrate Balanced Equation.
From testbook.com
Copper (II) Nitrate Formula Learn Structure, Properties, Uses Zinc Copper Ii Nitrate Balanced Equation The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. 1 atom in reagents and 2 atoms in products. The balanced equation will be calculated along with the. Multiply coefficient for zn by 2 2 zn +. $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. Check your learning. Zinc Copper Ii Nitrate Balanced Equation.
From meryes.weebly.com
Balanced chemical equation with state symbols calculator meryes Zinc Copper Ii Nitrate Balanced Equation The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. The numbers of n and o atoms on either side of the equation are now equal, and so the equation is. Zinc Copper Ii Nitrate Balanced Equation.
From www.youtube.com
How to Balance Zn(NO3)2 = ZnO + NO2 + O2 (Zinc Nitrate Zinc Copper Ii Nitrate Balanced Equation In order to balance zn on both sides we: Zn(s) + cu(n o3)2(aq) → zn(n o3)2(aq) + cu(s) explanation: The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Multiply coefficient for zn by 2 2 zn +. The balanced equation will appear. 1 atom in. Zinc Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED 16. Write complete balanced formula equation for the following Zinc Copper Ii Nitrate Balanced Equation The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. Multiply coefficient for zn by 2 2 zn +. $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. Zinc is a more active metal than copper (it lies higher up the. The balanced equation will appear. The equation above indicates that one mole of solid copper is reacting. Zinc Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED 1. Write the balanced chemical equation for the reaction of Zinc Copper Ii Nitrate Balanced Equation Zn(s) + cu(n o3)2(aq) → zn(n o3)2(aq) + cu(s) explanation: Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Check your learning write a. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. Zinc Copper Ii Nitrate Balanced Equation.
From slideplayer.com
Balancing Equations/ Translating Equations Practice ppt download Zinc Copper Ii Nitrate Balanced Equation The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. 1 atom in reagents and 2 atoms in products. $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. The balanced equation will appear. Enter an equation of an ionic chemical equation and press the balance button. The numbers of. Zinc Copper Ii Nitrate Balanced Equation.
From www.numerade.com
Using the activity series (Table 4.5), write balanced chemical Zinc Copper Ii Nitrate Balanced Equation Check your learning write a. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Multiply coefficient for zn by 2 2 zn +. The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. Zinc is a more active metal than copper. Zinc Copper Ii Nitrate Balanced Equation.
From www.chegg.com
Solved VI. Write formula equations and balance with Zinc Copper Ii Nitrate Balanced Equation The balanced equation will be calculated along with the. Check your learning write a. $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. The numbers of n and o atoms on either side of the equation are now equal, and so the equation is balanced. Zn(s) + cu(n o3)2(aq) → zn(n o3)2(aq) + cu(s) explanation: The equation above indicates that one mole of. Zinc Copper Ii Nitrate Balanced Equation.
From www.slideserve.com
PPT Balancing Chemical Reactions PowerPoint Presentation, free Zinc Copper Ii Nitrate Balanced Equation The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. In order to balance zn on both sides we: Zinc is a more active metal than copper (it lies higher up the. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. $zn(s). Zinc Copper Ii Nitrate Balanced Equation.
From www.coursehero.com
[Solved] Suppose 0.0615g of copper(II) nitrate is dissolved in 50.mL of Zinc Copper Ii Nitrate Balanced Equation The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. The balanced equation will be calculated along with the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Enter an equation of an ionic. Zinc Copper Ii Nitrate Balanced Equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation Zinc Copper Ii Nitrate Balanced Equation 1 atom in reagents and 2 atoms in products. Multiply coefficient for zn by 2 2 zn +. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. The equation above indicates that one mole of solid copper is reacting. Zinc Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED Give the complete ionic equation for the reaction (if any) that Zinc Copper Ii Nitrate Balanced Equation Multiply coefficient for zn by 2 2 zn +. The balanced equation will be calculated along with the. $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. The balanced equation will appear. 1 atom in reagents and 2 atoms in products. In order to balance zn on both sides we: Zinc is a more active metal than copper (it lies higher up the.. Zinc Copper Ii Nitrate Balanced Equation.
From www.chegg.com
Solved 761. Zinc metal reacts with aqueous copper(II) Zinc Copper Ii Nitrate Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Multiply coefficient for zn by 2 2 zn +. 1 atom in reagents and. Zinc Copper Ii Nitrate Balanced Equation.
From www.chegg.com
Solved Write the balanced equation for the reaction of zinc Zinc Copper Ii Nitrate Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will appear. The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. Check your learning write a. 1 atom in reagents and 2 atoms in products. The numbers of n and o atoms on either side of the equation. Zinc Copper Ii Nitrate Balanced Equation.
From www.meritnation.com
Write balanced chemical equations for the conversions A,B C A Copper Zinc Copper Ii Nitrate Balanced Equation Check your learning write a. $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. Multiply coefficient for zn by 2 2 zn +. Zinc is a more active metal than copper (it lies higher up the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. 1 atom in reagents and 2 atoms in products. The. Zinc Copper Ii Nitrate Balanced Equation.
From www.youtube.com
Equation for Zn(NO3)2 + H2O (Zinc nitrate + Water) YouTube Zinc Copper Ii Nitrate Balanced Equation 1 atom in reagents and 2 atoms in products. $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Multiply coefficient for zn by 2 2 zn +. The chemical equation for the reaction of zinc with copper (ii) nitrate. Zinc Copper Ii Nitrate Balanced Equation.
From www.youtube.com
AgNO3+Zn→Ag +Zn(NO3)2 Balanced EquationSilver nitrate+Zinc =Silver Zinc Copper Ii Nitrate Balanced Equation In order to balance zn on both sides we: $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. The balanced equation will appear. The numbers of n and o atoms on either side of the equation are now equal, and so the equation is balanced. Multiply coefficient for. Zinc Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for the singlereplacement oxidation Zinc Copper Ii Nitrate Balanced Equation Check your learning write a. The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. Enter an equation of an ionic chemical equation and press the balance button. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Zn(s) + cu(n. Zinc Copper Ii Nitrate Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Na2S + Zn(NO3)2 = NaNO3 + ZnS Zinc Copper Ii Nitrate Balanced Equation Zinc is a more active metal than copper (it lies higher up the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Check your learning write a. The. Zinc Copper Ii Nitrate Balanced Equation.
From www.youtube.com
How to Balance Zn + CuSO4 = Cu + ZnSO4 (Zinc plus Copper (II) Sulfate Zinc Copper Ii Nitrate Balanced Equation $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. In order to balance zn on both sides we: The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. 1 atom in reagents. Zinc Copper Ii Nitrate Balanced Equation.
From www.chegg.com
Solved (20) Write a balanced chemical equation for each of Zinc Copper Ii Nitrate Balanced Equation The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. Check your learning write a. 1 atom in reagents and 2 atoms in products. Zinc is a more active metal than copper (it lies higher up the. The balanced equation will appear. The numbers of n and o atoms on either side of the equation. Zinc Copper Ii Nitrate Balanced Equation.
From slideplayer.com
Redox Reactions and electrochemical cells ppt download Zinc Copper Ii Nitrate Balanced Equation 1 atom in reagents and 2 atoms in products. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Zn(s) + cu(n o3)2(aq) → zn(n o3)2(aq) + cu(s) explanation: $zn(s) + cu{(n{o_3})_2}(aq) \to zn{(n{o_3})_2}(aq) +. To balance a chemical equation, enter an equation of a chemical. Zinc Copper Ii Nitrate Balanced Equation.
From www.chegg.com
Solved What is the net ionic equation for the reaction of Zinc Copper Ii Nitrate Balanced Equation The chemical equation for the reaction of zinc with copper (ii) nitrate is shown below. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. In order to balance zn on both sides we: The balanced equation will be calculated along with the. To balance a. Zinc Copper Ii Nitrate Balanced Equation.
From www.chegg.com
Solved zinc metal is added to a solution of copper (II) Zinc Copper Ii Nitrate Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. Zinc is a more active metal than copper (it lies higher up the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Check your learning write a. The balanced equation will be. Zinc Copper Ii Nitrate Balanced Equation.