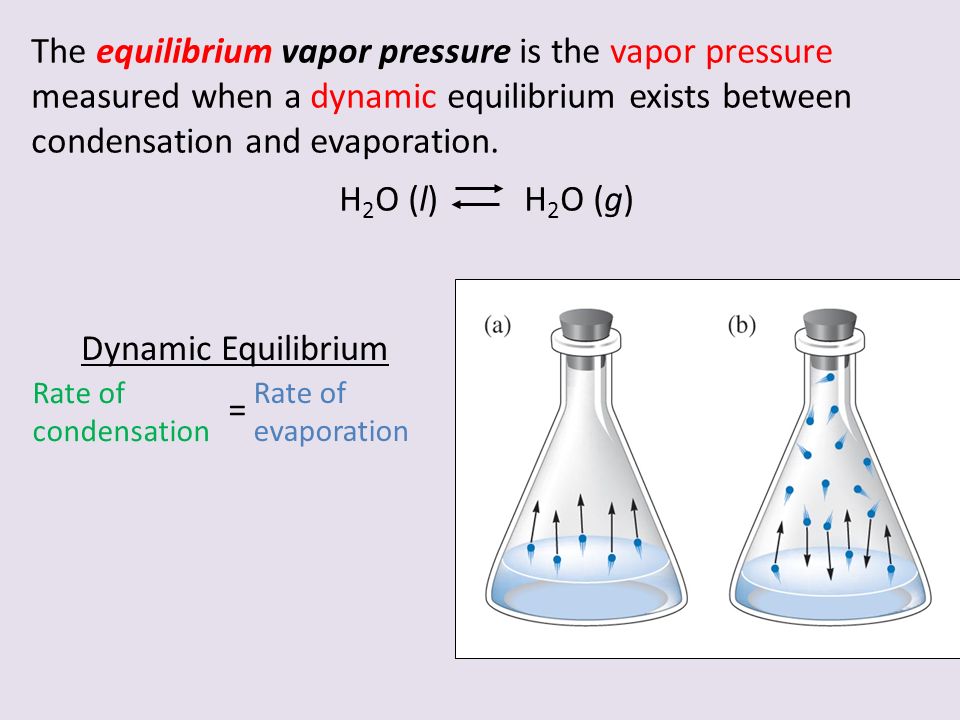
Liquid Evaporation And Vapor Pressure . Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. If we have an open container of. That is, the pressure of the vapor resulting from. The amount of vapor above the. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature.
from socratic.org
When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. The amount of vapor above the. The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. That is, the pressure of the vapor resulting from. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. If we have an open container of.
What do we mean by a dynamic equilibrium? Can you describe how the
Liquid Evaporation And Vapor Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. The amount of vapor above the. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. If we have an open container of. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. That is, the pressure of the vapor resulting from.
From www.peoi.org
Chapitre 10 Section C Properties of Liquids Liquid Evaporation And Vapor Pressure When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. That is, the pressure of the vapor resulting from. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. Vapor pressure is defined as the. Liquid Evaporation And Vapor Pressure.
From vacaero.com
Evaporation Liquid Evaporation And Vapor Pressure The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. The vapor pressure of a liquid is the equilibrium pressure of a vapor above. Liquid Evaporation And Vapor Pressure.
From www.youtube.com
Vapor Pressure, Equilibrium Vapor Pressure, and Relative Humidity YouTube Liquid Evaporation And Vapor Pressure That is, the pressure of the vapor resulting from. The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. Vapor pressure or equilibrium vapor. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download Liquid Evaporation And Vapor Pressure Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. The vapor pressure of a liquid is the equilibrium pressure of a. Liquid Evaporation And Vapor Pressure.
From chemistryskills.com
Define and explain vapour pressure Chemistry Skills Liquid Evaporation And Vapor Pressure If we have an open container of. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. Vapor pressure is defined as the partial pressure of a. Liquid Evaporation And Vapor Pressure.
From saylordotorg.github.io
Vapor Pressure Liquid Evaporation And Vapor Pressure The amount of vapor above the. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Liquid Evaporation And Vapor Pressure When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. If we have an open container of. The amount of vapor above the. The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. Vapor. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free Liquid Evaporation And Vapor Pressure That is, the pressure of the vapor resulting from. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. When a liquid is heated, its molecules obtain. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Liquid Evaporation And Vapor Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The amount of vapor above the. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with. Liquid Evaporation And Vapor Pressure.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Liquid Evaporation And Vapor Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. Vapor pressure is defined as the partial pressure of a substance in. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT To learn about dipoledipole, hydrogen bonding and London Liquid Evaporation And Vapor Pressure The amount of vapor above the. Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. Vapor pressure is defined as. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT Unit 13 Liquids and Solids PowerPoint Presentation, free Liquid Evaporation And Vapor Pressure The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. That is, the pressure of the vapor resulting from. When a liquid is heated,. Liquid Evaporation And Vapor Pressure.
From socratic.org
What do we mean by a dynamic equilibrium? Can you describe how the Liquid Evaporation And Vapor Pressure The amount of vapor above the. If we have an open container of. The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. Vapor pressure or equilibrium vapor pressure. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT Unit 13 Liquids and Solids PowerPoint Presentation, free Liquid Evaporation And Vapor Pressure The amount of vapor above the. If we have an open container of. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. Vapor pressure is. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free Liquid Evaporation And Vapor Pressure If we have an open container of. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. When a liquid is heated, its molecules obtain sufficient kinetic energy to. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free Liquid Evaporation And Vapor Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the. Liquid Evaporation And Vapor Pressure.
From pharmacyscope.com
Vapour pressure Pharmacy Scope Liquid Evaporation And Vapor Pressure Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); If we have an open container of. Vapor pressure is defined as the partial pressure of a substance in the gas phase. Liquid Evaporation And Vapor Pressure.
From www.peoi.org
Chapter 10 Section C Properties of Liquids Liquid Evaporation And Vapor Pressure The amount of vapor above the. The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. When a liquid is heated, its molecules obtain. Liquid Evaporation And Vapor Pressure.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Liquid Evaporation And Vapor Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the. Liquid Evaporation And Vapor Pressure.
From www.slideshare.net
Chapter7 Liquid Evaporation And Vapor Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The amount of vapor above the. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT LIQUIDS & SOLIDS PowerPoint Presentation, free download ID2511369 Liquid Evaporation And Vapor Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The amount of vapor above the. That is, the pressure of the vapor resulting from. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. Vapor pressure. Liquid Evaporation And Vapor Pressure.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Liquid Evaporation And Vapor Pressure When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. If we have an open container of. The vapor pressure. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 Liquid Evaporation And Vapor Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The amount of vapor above the. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT Liquids & Vapor Pressure PowerPoint Presentation ID4196264 Liquid Evaporation And Vapor Pressure That is, the pressure of the vapor resulting from. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The vapor pressure of a liquid is the. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT Unit 13 Liquids and Solids PowerPoint Presentation, free Liquid Evaporation And Vapor Pressure When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. The vapor pressure of a liquid is the equilibrium pressure. Liquid Evaporation And Vapor Pressure.
From serc.carleton.edu
Phase Rule Liquid Evaporation And Vapor Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. Vapor pressure is defined as the partial pressure of a gas in. Liquid Evaporation And Vapor Pressure.
From www.expii.com
Vapor Pressure — Definition & Overview Expii Liquid Evaporation And Vapor Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. That is, the pressure of the vapor resulting from. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and they escape into the. Vapor pressure is. Liquid Evaporation And Vapor Pressure.
From saylordotorg.github.io
Vapor Pressure Liquid Evaporation And Vapor Pressure Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. If we have an open container of. The vapor pressure of a. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free Liquid Evaporation And Vapor Pressure If we have an open container of. Vapor pressure is defined as the partial pressure of a gas in equilibrium with its liquid at a constant temperature. That is, the pressure of the vapor resulting from. The amount of vapor above the. The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of. Liquid Evaporation And Vapor Pressure.
From www.researchgate.net
Vapourliquid equilibrium of ethanolwater showing distillation steps Liquid Evaporation And Vapor Pressure If we have an open container of. The amount of vapor above the. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free Liquid Evaporation And Vapor Pressure That is, the pressure of the vapor resulting from. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. Vapor pressure is defined as the partial pressure of a substance in. Liquid Evaporation And Vapor Pressure.
From www.youtube.com
Chemistry 8.3 Evaporation and Vapor Pressure YouTube Liquid Evaporation And Vapor Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); If we have an open container of. The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic. Liquid Evaporation And Vapor Pressure.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Liquid Evaporation And Vapor Pressure Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. When a liquid is heated, its molecules obtain sufficient. Liquid Evaporation And Vapor Pressure.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Liquid Evaporation And Vapor Pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. That is, the pressure of the vapor resulting from. If we have an open container of. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and. Liquid Evaporation And Vapor Pressure.
From www.slideshare.net
Chapter 11 Properties of Solutions Liquid Evaporation And Vapor Pressure The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); The vapor pressure of a liquid is the equilibrium pressure exerted by the vapor on the surface of the liquid phase. When a liquid is heated, its molecules obtain sufficient kinetic energy to overcome the forces holding them in the liquid and. Liquid Evaporation And Vapor Pressure.