Medical Device Manufacturing Date Definition . If there's no expiry date but the device has a definite shelf life, the manufacturing date would be required so that the user can. The medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in.
from medtechintelligence.com
If there's no expiry date but the device has a definite shelf life, the manufacturing date would be required so that the user can. The medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a.
Column Compliance Date Approaching for FDA Unique Device Identifiers
Medical Device Manufacturing Date Definition Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. If there's no expiry date but the device has a definite shelf life, the manufacturing date would be required so that the user can. ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. The medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the.
From medtechintelligence.com
Column Compliance Date Approaching for FDA Unique Device Identifiers Medical Device Manufacturing Date Definition Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. ‘medical device’ means any. Medical Device Manufacturing Date Definition.
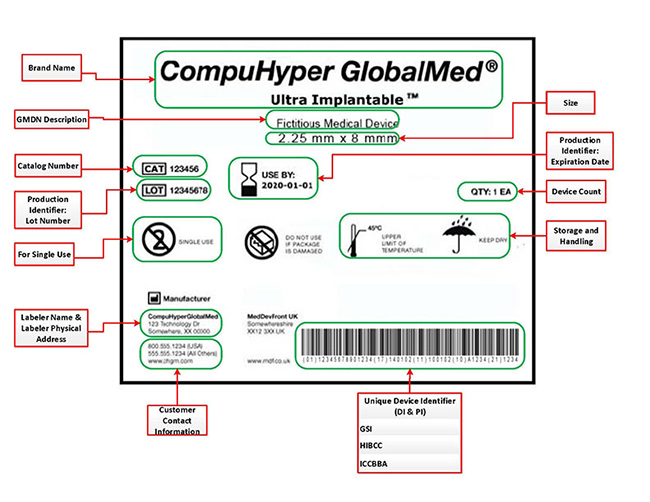
From mungfali.com
Medical Device Labeling Symbols Medical Device Manufacturing Date Definition If there's no expiry date but the device has a definite shelf life, the manufacturing date would be required so that the user can. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material. Medical Device Manufacturing Date Definition.
From www.shutterstock.com
Date Manufacture Medical Devices Marking Medical Stock Vector (Royalty Medical Device Manufacturing Date Definition Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. If there's no expiry date but the device has a definite shelf life, the manufacturing date would be required so that the user can. The medical devices regulations 2002 is up to date with all. Medical Device Manufacturing Date Definition.
From pharmaknowl.com
SFDA Labelling Requirements PharmaKnowl Medical Device Manufacturing Date Definition Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. The medical devices regulations. Medical Device Manufacturing Date Definition.
From pivotint.co.uk
Medical Device Manufacturing From Concept to Market » Pivot UK Medical Device Manufacturing Date Definition ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. If there's no expiry date but the device has a definite shelf life, the manufacturing date would be required so that the user can. Whenever the label of a medical device includes a printed expiration date,. Medical Device Manufacturing Date Definition.
From ambitiousmares.blogspot.com
34 Medical Device Label Symbols Labels Design Ideas 2020 Medical Device Manufacturing Date Definition Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. If there's no expiry date but the device has a definite shelf life, the manufacturing date would be required so that the user can. Whenever the label of a. Medical Device Manufacturing Date Definition.
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download Medical Device Manufacturing Date Definition ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. If there's no expiry date but the device has a definite shelf life,. Medical Device Manufacturing Date Definition.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Medical Device Manufacturing Date Definition So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. The medical devices regulations 2002 is up to date with all changes known. Medical Device Manufacturing Date Definition.
From medicaldevicehq.com
UDI requirements for medical device manufacturers in the EU Medical Medical Device Manufacturing Date Definition If there's no expiry date but the device has a definite shelf life, the manufacturing date would be required so that the user can. So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. Whenever the label of a medical device includes a printed expiration date, date. Medical Device Manufacturing Date Definition.
From www.vectorstock.com
Date manufacture medical devices marking Vector Image Medical Device Manufacturing Date Definition The medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. If there's no expiry date but. Medical Device Manufacturing Date Definition.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Medical Device Manufacturing Date Definition Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or. Medical Device Manufacturing Date Definition.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Manufacturing Date Definition Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. If there's no expiry date but the device has a definite shelf life, the manufacturing date would be required so that the user can. ‘medical device’ means any instrument,. Medical Device Manufacturing Date Definition.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Manufacturing Date Definition If there's no expiry date but the device has a definite shelf life, the manufacturing date would be required so that the user can. ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. The medical devices regulations 2002 is up to date with all changes. Medical Device Manufacturing Date Definition.
From exogphupj.blob.core.windows.net
Medical Device Labelling Tga at William Maurer blog Medical Device Manufacturing Date Definition Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. If there's no expiry. Medical Device Manufacturing Date Definition.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Medical Device Manufacturing Date Definition So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. If there's no expiry date but the device has a definite shelf life, the manufacturing date would be required so that the user can. The medical devices regulations 2002 is up to date with all changes known. Medical Device Manufacturing Date Definition.
From sri-healthcare.com
UDI Symbol Glossary Medical Device Manufacturing Date Definition ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. Whenever the label of a medical device includes a printed expiration date, date. Medical Device Manufacturing Date Definition.
From ar.inspiredpencil.com
Medical Signs And Symbols Medical Device Manufacturing Date Definition So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. The medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. If there's no expiry date but the device has a definite shelf life, the. Medical Device Manufacturing Date Definition.
From www.reddit.com
Medical quipment symbols demystified. r/TacticalMedicine Medical Device Manufacturing Date Definition The medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. If there's no expiry date but the device has a definite shelf life, the. Medical Device Manufacturing Date Definition.
From mungfali.com
Medical Device Labeling Symbols Medical Device Manufacturing Date Definition Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. The medical devices regulations 2002 is. Medical Device Manufacturing Date Definition.
From www.molnlycke.us
Regulatory support and product information Mölnlycke Medical Device Manufacturing Date Definition The medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. If there's no expiry date but the device has a definite shelf. Medical Device Manufacturing Date Definition.
From mungfali.com
Medical Device Labeling Symbols Medical Device Manufacturing Date Definition So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. The medical devices regulations 2002 is up to date with all changes known. Medical Device Manufacturing Date Definition.
From easymedicaldevice.com
How to write a Declaration of Conformity? (MDR and IVDR) Medical Medical Device Manufacturing Date Definition The medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. So for medical device abc we produce, the manufacturing date is set. Medical Device Manufacturing Date Definition.
From www.congress-intercultural.eu
Understanding The Different Types Of Manufacturing Date, 47 OFF Medical Device Manufacturing Date Definition So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. The medical devices regulations 2002 is up to date with all changes known. Medical Device Manufacturing Date Definition.
From www.shutterstock.com
3,010 Manufacture date Images, Stock Photos & Vectors Shutterstock Medical Device Manufacturing Date Definition The medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. If there's no expiry date but the device has a definite shelf life, the manufacturing date would be required so that the user can. So for medical device abc we produce, the manufacturing date is set by. Medical Device Manufacturing Date Definition.
From www.datumdental.com
Symbols Glossary Medical Devices Datum Dental Medical Device Manufacturing Date Definition Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. The medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or. Medical Device Manufacturing Date Definition.
From www.dreamstime.com
Date of Manufacture of Medical Devices. Marking of Medical Products Medical Device Manufacturing Date Definition ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. The medical devices regulations 2002 is up to date with all. Medical Device Manufacturing Date Definition.
From www.slideshare.net
Symbols Commonly Used in Medical Device Packaging and Labeling Medical Device Manufacturing Date Definition ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. The medical devices regulations 2002. Medical Device Manufacturing Date Definition.
From www.dreamstime.com
Manufacturing Date and Expiry Date. Stock Image Image of concept Medical Device Manufacturing Date Definition If there's no expiry date but the device has a definite shelf life, the manufacturing date would be required so that the user can. ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. The medical devices regulations 2002 is up to date with all changes. Medical Device Manufacturing Date Definition.
From www.alamy.com
Details of expiry date and manufacturing date on product label Stock Medical Device Manufacturing Date Definition So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the attention of the user of the. Whenever the label of a medical. Medical Device Manufacturing Date Definition.
From khatabook.com
What Is MFG Date? MFG Date Full Form, Process, Types & Example Medical Device Manufacturing Date Definition ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. If there's no expiry date but the device has a definite shelf life,. Medical Device Manufacturing Date Definition.
From exyrvtbcz.blob.core.windows.net
Symbols In Medical Device Labeling at Jillian Bundy blog Medical Device Manufacturing Date Definition If there's no expiry date but the device has a definite shelf life, the manufacturing date would be required so that the user can. The medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other. Medical Device Manufacturing Date Definition.
From exoaqfrfb.blob.core.windows.net
Medical Devices Manufacturing Companies In Netherlands at Bernadette Medical Device Manufacturing Date Definition ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. So for medical device abc we produce, the manufacturing date is. Medical Device Manufacturing Date Definition.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Manufacturing Date Definition The medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. ‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in. Whenever the label of a medical device includes a printed expiration date, date. Medical Device Manufacturing Date Definition.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Manufacturing Date Definition Whenever the label of a medical device includes a printed expiration date, date of manufacture, or any other date intended to be brought to the. So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. If there's no expiry date but the device has a definite shelf. Medical Device Manufacturing Date Definition.
From mungfali.com
Medical Label Symbols Medical Device Manufacturing Date Definition So for medical device abc we produce, the manufacturing date is set by the erp system the first time the product sees a. The medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. If there's no expiry date but the device has a definite shelf life, the. Medical Device Manufacturing Date Definition.