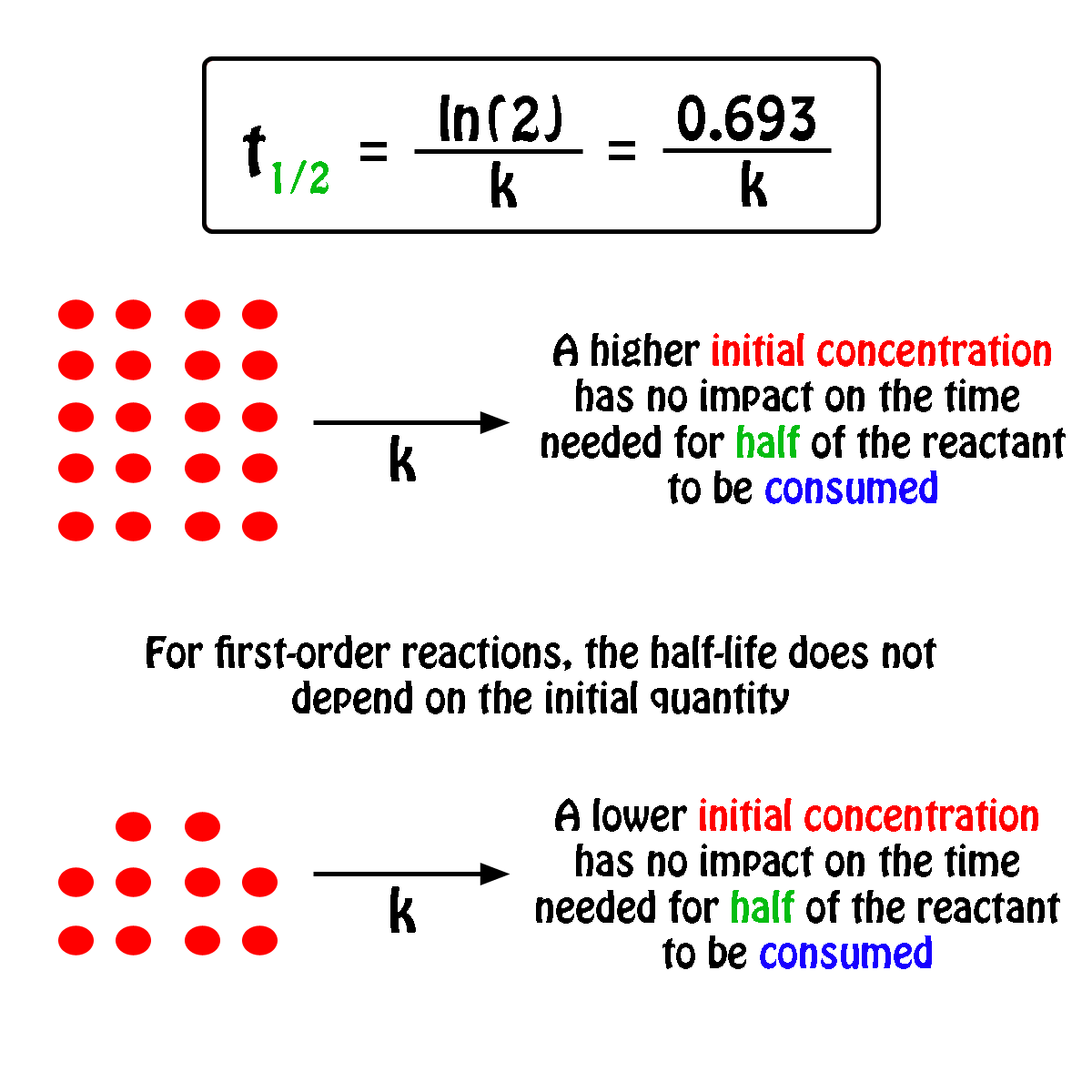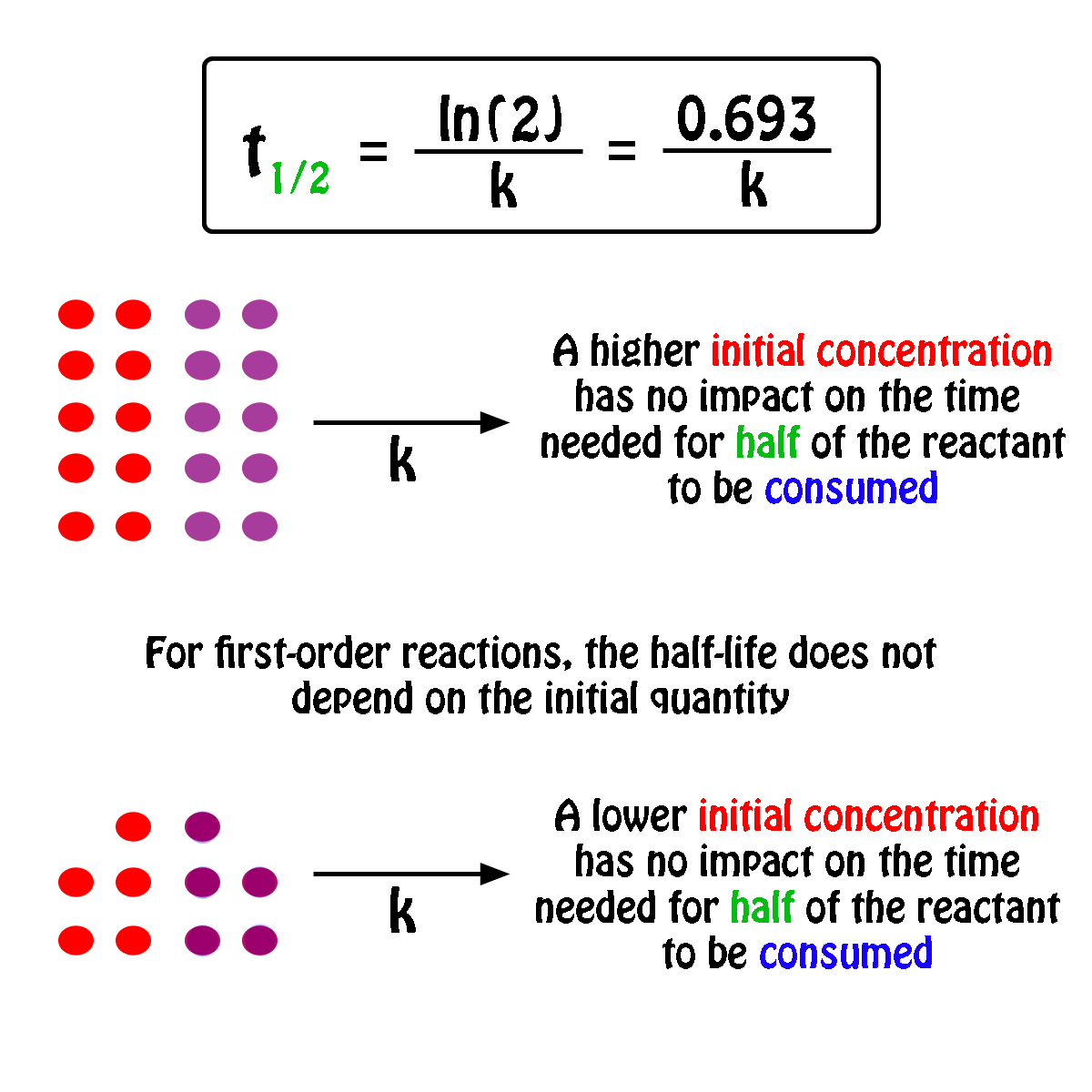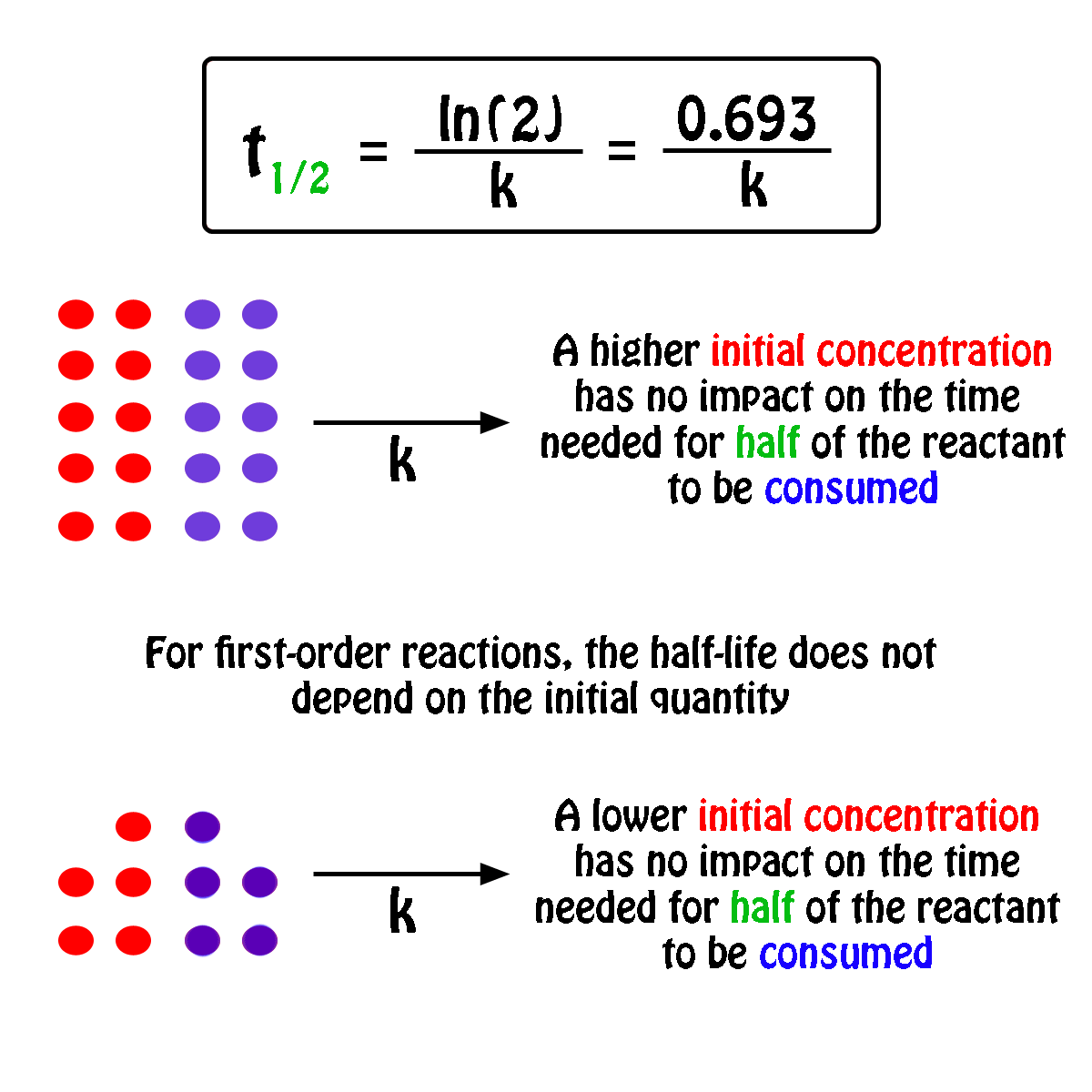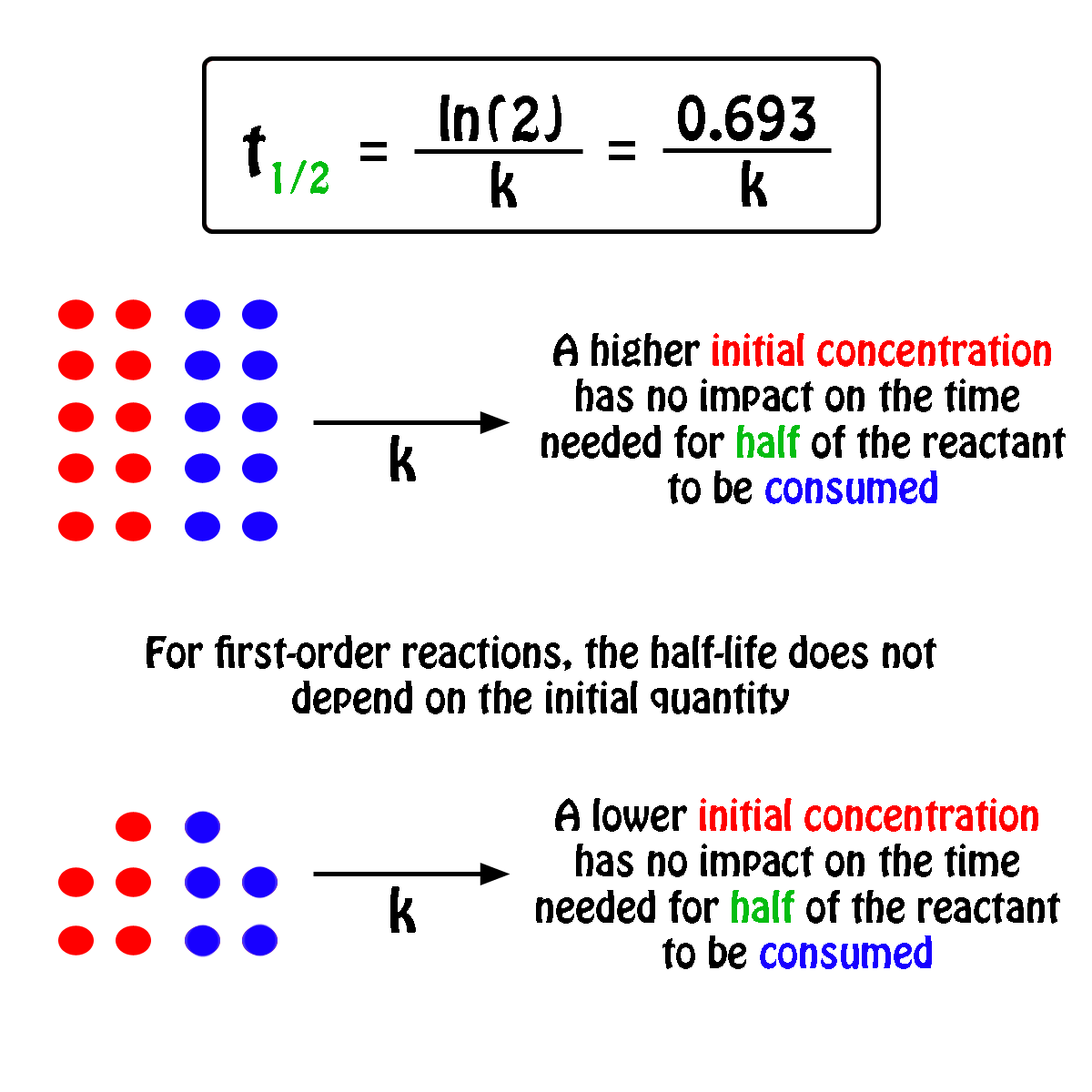
Rate Constant First Order Reaction Value . More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. In other words, if the concentration is doubled, the reaction rate is. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws.
from socratic.org
Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. In other words, if the concentration is doubled, the reaction rate is. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws.
What is the halflife of a firstorder reaction with a rate constant of
Rate Constant First Order Reaction Value Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. In other words, if the concentration is doubled, the reaction rate is. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws.
From byjus.com
a first ordre rection has a rate constant of 2.303 into 10 power minus Rate Constant First Order Reaction Value Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. In. Rate Constant First Order Reaction Value.
From mavink.com
First Order Reaction Plot Rate Constant First Order Reaction Value More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. In other words, if the concentration is doubled, the reaction rate is. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. Differential rate laws are generally used to describe what is occurring. Rate Constant First Order Reaction Value.
From chemistnotes.com
First Order Reaction definition, example, half life period Chemistry Rate Constant First Order Reaction Value More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. In other words, if the concentration is doubled, the reaction rate is. Differential rate laws are generally used to describe what is occurring. Rate Constant First Order Reaction Value.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Rate Constant First Order Reaction Value In other words, if the concentration is doubled, the reaction rate is. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Integrated rate expressions can be used to. Rate Constant First Order Reaction Value.
From chemistryguru.com.sg
Rate Equation and Order of Reaction Rate Constant First Order Reaction Value More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. In other words, if the concentration is doubled, the reaction rate is. Integrated rate expressions can be used to. Rate Constant First Order Reaction Value.
From www.brainkart.com
Rate equation for first order reactions Rate Constant First Order Reaction Value More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. In other words, if the concentration is doubled, the reaction rate is. Differential rate laws are generally used to describe what is occurring. Rate Constant First Order Reaction Value.
From www.numerade.com
SOLVED(a) A certain firstorder reaction has a rate constant of 2.75 × Rate Constant First Order Reaction Value In other words, if the concentration is doubled, the reaction rate is. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. Integrated rate expressions can be used to. Rate Constant First Order Reaction Value.
From www.toppr.com
t1/2 for a first order reaction is 6.93 s , the value of rate constant Rate Constant First Order Reaction Value In other words, if the concentration is doubled, the reaction rate is. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. Integrated rate expressions can be used to. Rate Constant First Order Reaction Value.
From byjus.com
The rate constant for a first order reaction at 300 deg;celsius for Rate Constant First Order Reaction Value In other words, if the concentration is doubled, the reaction rate is. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. More generally speaking, the units for the rate. Rate Constant First Order Reaction Value.
From www.youtube.com
The rate constant for a first order reaction six times when the Rate Constant First Order Reaction Value In other words, if the concentration is doubled, the reaction rate is. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. Differential rate laws are generally used to describe what is occurring. Rate Constant First Order Reaction Value.
From galldemax10.netlify.app
First Order Integrated Rate Law Equation Gallery Dedemax Rate Constant First Order Reaction Value In other words, if the concentration is doubled, the reaction rate is. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. More generally speaking, the units for the rate. Rate Constant First Order Reaction Value.
From www.youtube.com
Integrated Rate Equation for first Order Reaction First Order Rate Constant First Order Reaction Value Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. In. Rate Constant First Order Reaction Value.
From 2012books.lardbucket.org
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders Rate Constant First Order Reaction Value Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. In other words, if the concentration is doubled, the reaction rate is. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Differential rate laws are generally used to describe what is occurring. Rate Constant First Order Reaction Value.
From www.youtube.com
Quickvideo Calculating rate constant from a firstorder reaction Rate Constant First Order Reaction Value More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. In other words, if the concentration is doubled, the reaction rate is. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. Integrated rate expressions can be used to. Rate Constant First Order Reaction Value.
From socratic.org
What is the halflife of a firstorder reaction with a rate constant of Rate Constant First Order Reaction Value Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. In. Rate Constant First Order Reaction Value.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial Rate Constant First Order Reaction Value More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. In. Rate Constant First Order Reaction Value.
From www.sliderbase.com
Rate Laws Presentation Chemistry Rate Constant First Order Reaction Value More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. In other words, if the concentration is doubled, the reaction rate is. Differential rate laws are generally used to describe what is occurring. Rate Constant First Order Reaction Value.
From www.brainkart.com
Rate equation for first order reactions Rate Constant First Order Reaction Value In other words, if the concentration is doubled, the reaction rate is. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. More generally speaking, the units for the rate. Rate Constant First Order Reaction Value.
From www.chegg.com
Solved What is the rate constant of a firstorder reaction Rate Constant First Order Reaction Value More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. In. Rate Constant First Order Reaction Value.
From study.com
First Order Reaction & Rate Law Definition, Equation & Examples Rate Constant First Order Reaction Value Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. In other words, if the concentration is doubled, the reaction rate is. Integrated rate expressions can be used to. Rate Constant First Order Reaction Value.
From general.chemistrysteps.com
Rate Law and Reaction Order Chemistry Steps Rate Constant First Order Reaction Value In other words, if the concentration is doubled, the reaction rate is. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. Integrated rate expressions can be used to. Rate Constant First Order Reaction Value.
From www.coursehero.com
[Solved] A certain firstorder reaction has a rate constant of Rate Constant First Order Reaction Value In other words, if the concentration is doubled, the reaction rate is. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. Differential rate laws are generally used to describe what is occurring. Rate Constant First Order Reaction Value.
From www.youtube.com
first order reaction rate calculation example YouTube Rate Constant First Order Reaction Value Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. In. Rate Constant First Order Reaction Value.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID6090305 Rate Constant First Order Reaction Value More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. In. Rate Constant First Order Reaction Value.
From www.researchgate.net
How to calculate rate constant for first order reaction? ResearchGate Rate Constant First Order Reaction Value In other words, if the concentration is doubled, the reaction rate is. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Differential rate laws are generally used to describe what is occurring. Rate Constant First Order Reaction Value.
From www.toppr.com
For a first order reaction rate constant is 1 × 10 ^ 5sec ^ 1 Rate Constant First Order Reaction Value Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. In. Rate Constant First Order Reaction Value.
From newbedev.com
Chemistry Formula for rate constant for the first order reaction Rate Constant First Order Reaction Value Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. In. Rate Constant First Order Reaction Value.
From www.vrogue.co
First Order Reaction Units vrogue.co Rate Constant First Order Reaction Value In other words, if the concentration is doubled, the reaction rate is. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. Integrated rate expressions can be used to. Rate Constant First Order Reaction Value.
From general.chemistrysteps.com
FirstOrder Reactions Chemistry Steps Rate Constant First Order Reaction Value In other words, if the concentration is doubled, the reaction rate is. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. More generally speaking, the units for the rate. Rate Constant First Order Reaction Value.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID6090305 Rate Constant First Order Reaction Value More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. In. Rate Constant First Order Reaction Value.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID6630235 Rate Constant First Order Reaction Value Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. In other words, if the concentration is doubled, the reaction rate is. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. More generally speaking, the units for the rate. Rate Constant First Order Reaction Value.
From www.slideserve.com
PPT Chapter 15 Chemical The Rates of Chemical Reactions Rate Constant First Order Reaction Value More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. In other words, if the concentration is doubled, the reaction rate is. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. Differential rate laws are generally used to describe what is occurring. Rate Constant First Order Reaction Value.
From www.tessshebaylo.com
Derive Integrated Rate Equation For A First Order Gas Phase Reaction Rate Constant First Order Reaction Value More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. In other words, if the concentration is doubled, the reaction rate is. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. Integrated rate expressions can be used to. Rate Constant First Order Reaction Value.
From www.researchgate.net
Firstorder reaction rate constants (k obs ) and initial reaction rates Rate Constant First Order Reaction Value In other words, if the concentration is doubled, the reaction rate is. More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. Integrated rate expressions can be used to. Rate Constant First Order Reaction Value.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID6090305 Rate Constant First Order Reaction Value More generally speaking, the units for the rate constant for a reaction of order \( (m+n)\) are \(\ce{mol}^{1−(m+n)}\ce l^{(m+n)−1}\ce. Integrated rate expressions can be used to experimentally calculate the value of the rate constant of a reaction. In other words, if the concentration is doubled, the reaction rate is. Differential rate laws are generally used to describe what is occurring. Rate Constant First Order Reaction Value.