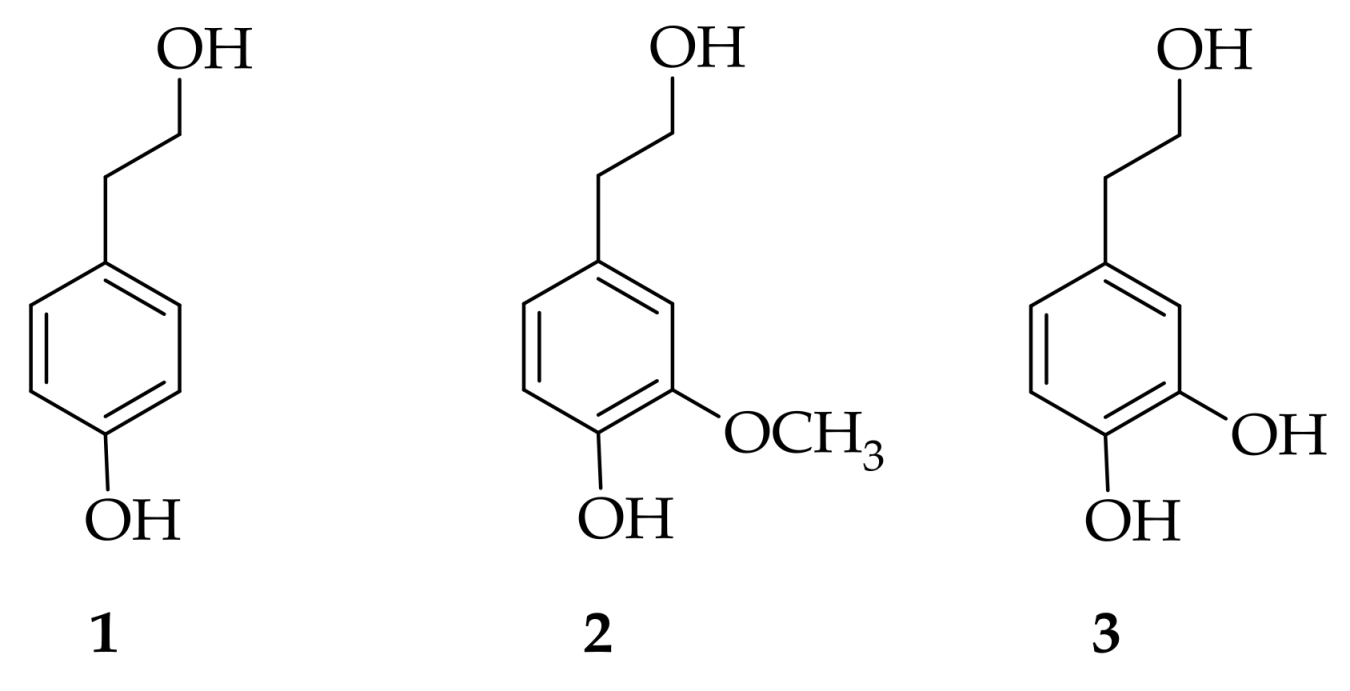
Lipophilic Esters . We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. Lipids, organic compounds, reaction products. New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of.
from www.mdpi.com
Lipids, organic compounds, reaction products. New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions.
Antioxidants Free FullText Synthesis of Lipophilic Esters of
Lipophilic Esters A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. Lipids, organic compounds, reaction products. A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions.
From www.abcam.com
Fluorescein octadecyl ester, Lipophilic fluorescent pH indicator (CAS Lipophilic Esters Lipids, organic compounds, reaction products. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. New lipophilic esters of. Lipophilic Esters.
From www.semanticscholar.org
Figure 1 from Lipophilic ester and amide derivatives of rosmarinic acid Lipophilic Esters Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact. Lipophilic Esters.
From pubs.acs.org
Efficient Enzymatic Synthesis of Lipophilic Phenolic Glycoside Azelaic Lipophilic Esters We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. New lipophilic esters of tyrosol, a naturally occurring phenol. Lipophilic Esters.
From www.researchgate.net
(PDF) Enzymatic Synthesis of Lipophilic Esters of Phenolic Compounds Lipophilic Esters Lipids, organic compounds, reaction products. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. A set of hydroxytyrosol conjugates with fatty acids at. Lipophilic Esters.
From www.mdpi.com
Antioxidants Free FullText Synthesis of Lipophilic Esters of Lipophilic Esters Lipids, organic compounds, reaction products. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. New lipophilic esters of tyrosol, a naturally occurring. Lipophilic Esters.
From www.slideserve.com
PPT Structure Activity Relationships of Local Anesthetics PowerPoint Lipophilic Esters Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect. Lipophilic Esters.
From www.slideserve.com
PPT Prodrugs PowerPoint Presentation, free download ID442444 Lipophilic Esters Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. A set of hydroxytyrosol conjugates with fatty acids at. Lipophilic Esters.
From www.researchgate.net
Various lipophilic molecules used for conjugation with oligonucleotides Lipophilic Esters Lipids, organic compounds, reaction products. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. A set of hydroxytyrosol conjugates with fatty acids at. Lipophilic Esters.
From www.semanticscholar.org
Figure 1 from Lipophilic ester and amide derivatives of rosmarinic acid Lipophilic Esters Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. We determined the interfacial molarities of. Lipophilic Esters.
From www.mdpi.com
Antioxidants Free FullText Synthesis of Lipophilic Esters of Lipophilic Esters A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. New lipophilic esters of tyrosol, a. Lipophilic Esters.
From www.academia.edu
(PDF) Enzymatic Synthesis of Lipophilic Esters of Phenolic Compounds Lipophilic Esters A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. We determined the interfacial. Lipophilic Esters.
From www.mdpi.com
Biomolecules Free FullText Enzymatic Synthesis of Lipophilic Lipophilic Esters New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. Lipids, organic compounds, reaction products. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of.. Lipophilic Esters.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Lipid Lipophilic Esters Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. Lipids, organic compounds, reaction products. A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. New. Lipophilic Esters.
From www.semanticscholar.org
Figure 1 from Lipophilic ester and amide derivatives of rosmarinic acid Lipophilic Esters A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and. Lipophilic Esters.
From europepmc.org
Hydrophilic and lipophilic characteristics of nonfatty acid moieties Lipophilic Esters Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. We determined the interfacial molarities. Lipophilic Esters.
From www.slideserve.com
PPT Structure Activity Relationships of Local Anesthetics PowerPoint Lipophilic Esters A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. Lipids, organic compounds, reaction. Lipophilic Esters.
From www.researchgate.net
(PDF) Transfer Investigations of Lipophilic Drugs from Lipid Lipophilic Esters Lipids, organic compounds, reaction products. A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. We. Lipophilic Esters.
From www.semanticscholar.org
Figure 1 from Lipophilic ester and amide derivatives of rosmarinic acid Lipophilic Esters A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to. Lipophilic Esters.
From chemistry-europe.onlinelibrary.wiley.com
Lignin Nanoparticles Support Lipase‐Tyrosinase Enzymatic Cascade in the Lipophilic Esters A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. Lipids, organic compounds, reaction products. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to. Lipophilic Esters.
From www.academia.edu
(PDF) Lipophilic Hydroxytyrosol Esters Fatty Acid Conjugates for Lipophilic Esters New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact. Lipophilic Esters.
From www.semanticscholar.org
Figure 1 from Lipophilic ester and amide derivatives of rosmarinic acid Lipophilic Esters A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and. Lipophilic Esters.
From www.semanticscholar.org
Figure 1 from Lipophilic activated ester prodrug approach for drug Lipophilic Esters New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. Lipids, organic compounds, reaction products. Pande and akoh prepared lipophilic tyrosyl esters. Lipophilic Esters.
From www.lifeder.com
Los 27 Ejemplos de Lípidos Más Importantes Lifeder Lipophilic Esters Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. We determined the interfacial molarities. Lipophilic Esters.
From www.researchgate.net
Model explaining the behavior of the esters 1219 within Lipophilic Esters New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. Lipids, organic compounds, reaction products. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild. Lipophilic Esters.
From www.researchgate.net
Characterization of bioreducible lipophilic poly(betaamino ester Lipophilic Esters We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. A set of hydroxytyrosol conjugates with fatty acids at. Lipophilic Esters.
From www.researchgate.net
Structures representative of the main aliphatic lipophilic compounds Lipophilic Esters New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact. Lipophilic Esters.
From chemistry-europe.onlinelibrary.wiley.com
Lignin Nanoparticles Support Lipase‐Tyrosinase Enzymatic Cascade in the Lipophilic Esters We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. A set of hydroxytyrosol conjugates with fatty acids at different molecular weights. Lipophilic Esters.
From www.researchgate.net
(PDF) Lipophilic Arginine Esters The Gateway to Preservatives without Lipophilic Esters Lipids, organic compounds, reaction products. A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. Pande and akoh prepared lipophilic tyrosyl esters with. Lipophilic Esters.
From www.researchgate.net
Schematic description of hydrophilic and lipophilic enzyme Lipophilic Esters Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. A set of hydroxytyrosol conjugates with fatty acids at. Lipophilic Esters.
From www.researchgate.net
Schematic representation of the difference between lipophilic and Lipophilic Esters Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. Lipids, organic compounds, reaction products.. Lipophilic Esters.
From www.medchemexpress.com
Fluorescein octadecyl ester Lipophilic Fluorescent Reagent Lipophilic Esters A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with. Lipophilic Esters.
From www.mdpi.com
Antioxidants Free FullText Synthesis of Lipophilic Esters of Lipophilic Esters New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. Lipids, organic compounds, reaction products. Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of.. Lipophilic Esters.
From docslib.org
Synthesis of Lipophilic Esters of Tyrosol, Homovanillyl Alcohol and Lipophilic Esters Lipids, organic compounds, reaction products. Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. We determined the interfacial molarities of the antioxidants, aos, hydroxytyrosol (ht), and ht fatty acid esters with chain lengths of 1 to 16 carbons in intact olive oil/water/tween 20 emulsions. Pande and akoh prepared. Lipophilic Esters.
From www.researchgate.net
Natural products fosmidomycin and FR900098, and their lipophilic esters Lipophilic Esters Pande and akoh prepared lipophilic tyrosyl esters with a carbon alkyl chain and different unsaturation degrees. A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. We determined the interfacial molarities of the antioxidants, aos,. Lipophilic Esters.
From www.researchgate.net
Structure of lipophilic cationexchanger (1)... Download Scientific Lipophilic Esters Ht esters, such as palmitate, oleate and linoleate, showed a greater capacity with respect to ht to prevent the generation of. A set of hydroxytyrosol conjugates with fatty acids at different molecular weights were synthesized under mild conditions. New lipophilic esters of tyrosol, a naturally occurring phenol with interesting biological properties, have been synthesized in good. Pande and akoh prepared. Lipophilic Esters.